The total number of contributing structures showing hyperconjugation (involving C−H bonds) for the following carbocation is
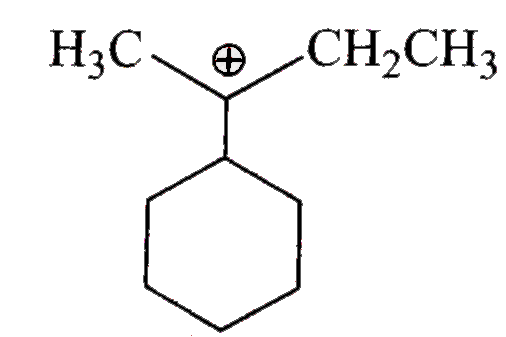
4
5
6
8
The correct Answer is:C
Step by step video & image solution for The total number of contributing structures showing hyperconjugation (involving C-H bonds) for the following carbocation is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
The standard EMF of the cell reaction
12Cu(s)+12Cl2(g)→12Cu2++Cl−
is 0.2 V. The value of △G0 will be
View Solution(i) The stability of carbocation is explain by delocalised structure of hyperconjugation.
(ii) The stability of carbocation is explain by drawing the resonance structure.
(iii) Hyperconjugation effect is (+) or (-)
(iv) Mesomeric effect is (+) or (-)
View Solution(i) The stability of carbocation is explain by delocalised structure of hyperconjugation. (ii) The stability of carbocation is explain by drawing the resonance structure. (iii) Hyperconjugation effect is (+) or (-) (iv) Mesomeric effect is (+) or (-)
View SolutionHyperconjugation describes the orbital interactions between the p-systems and the adjacent s-bond of the substituent group(s) in organic compounds. Hyperconjugation is also called as Baker and Nathen effect. The necessary and sufficient condition for the hyperconjugation are :
i) Compound should have at least on sp2 hybrid carbon of either alkene, carbocation or alkyl free radical.
ii) A-carbon with respect to sp2 hybrid carbon should have at least one hydrogen. Hyperconjugation are of three types: (i) s(C-H), p-conjugation.
(iii) s(C-H), positive charge conjugation
iv) s(C-H), odd electron conjugation
The hyperconjugation may be represented as
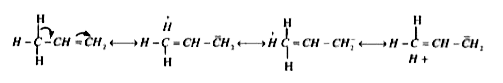
Number of resonating structures due to hyperconjugation = (n + 1) where n is the number of a-hydrogen.
Greater is the number of such forms, more is the stability of the species under considersation.
Which of the following carbocations will show highest number of Hyperconjugation forms?
View SolutionHyperconjugation OF Alkenes || Carbocation || Free Radicals || +H and -H Effect on Benzene
View SolutionHyperconjugation is defined as No bond resonance. The concept of hyperconjugation arose from the discovery of electron releasing pattern for alkyl groups. It involves sigma electrons of C-H bond. Greater the number of C-H bond ( alpha -hydrogen atom w.r.t. double bond) more will be hyperconjugative structures, more will be stability Heat of hydrogenation of alkene are affected by hyperconjugative effects. Which of the following is incorrect hyperconjugative structure?
View SolutionHyperconjugation is defined as No bond resonance. The concept of hyperconjugation arose from the discovery of electron releasing pattern for alkyl groups. It involves sigma electrons of C-H bond. Greater the number of C-H bond ( alpha -hydrogen atom w.r.t. double bond) more will be hyperconjugative structures, more will be stability Heat of hydrogenation of alkene are affected by hyperconjugative effects. Which of the following has highest heat of hydrogenation?
View SolutionThe total number of contributing structures showing hyperconjugation (involving C−H bonds) for the following carbocation is

View Solution
