The equilibrium constant for the following reaction is
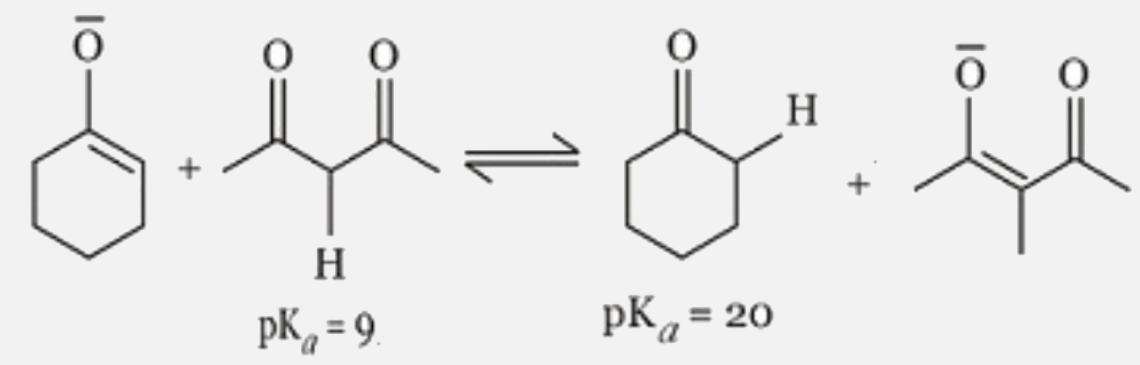
1011
10−11
1029
10−29
The correct Answer is:A
Step by step video & image solution for The equilibrium constant for the following reaction is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Which of the following converts the equilibrium constant of a reverse reaction to the equilibrium constant of an advance reaction?
View SolutionEquilibrium constant of a reverse reaction is more than the equilibrium constant of an advance reaction
View SolutionEquilibrium constant of a reverse reaction is more than the equilibrium constant of an advance reaction
View SolutionEquilibrium constant of a reverse reaction is more than the equilibrium constant of an advance reaction
View SolutionEquilibrium constant of a reverse reaction is more than the equilibrium constant of an advance reaction
View SolutionThe equilibrium constant of a reaction is 20.0. At equilibrium, the rate constant of forward reaction is 10.0. The rate constant for backward reaction is:
View SolutionThe equilibrium constant of a reaction is 300, if the volume of the reaction flask is tripled, the equilibrium constant will be
View SolutionThe equilibrium constant of a reaction is 20.0. At equilibrium, the reate constant of forward reaction is 10.0. The rate constant for backward reaction is :
View Solution

