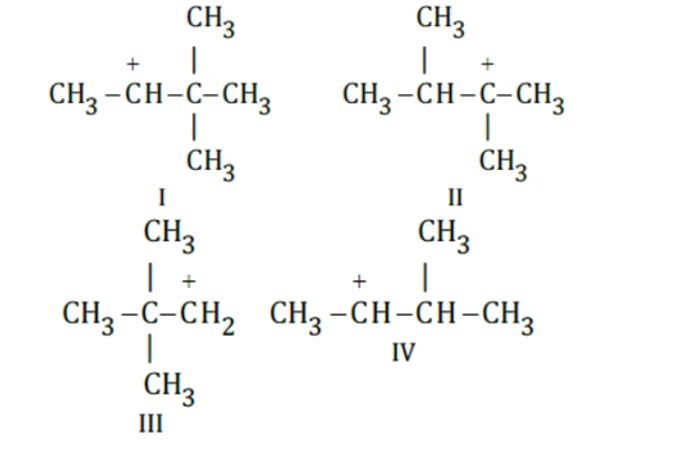Which of the following carbocations would not likely rearrange to more stable carbocation ?
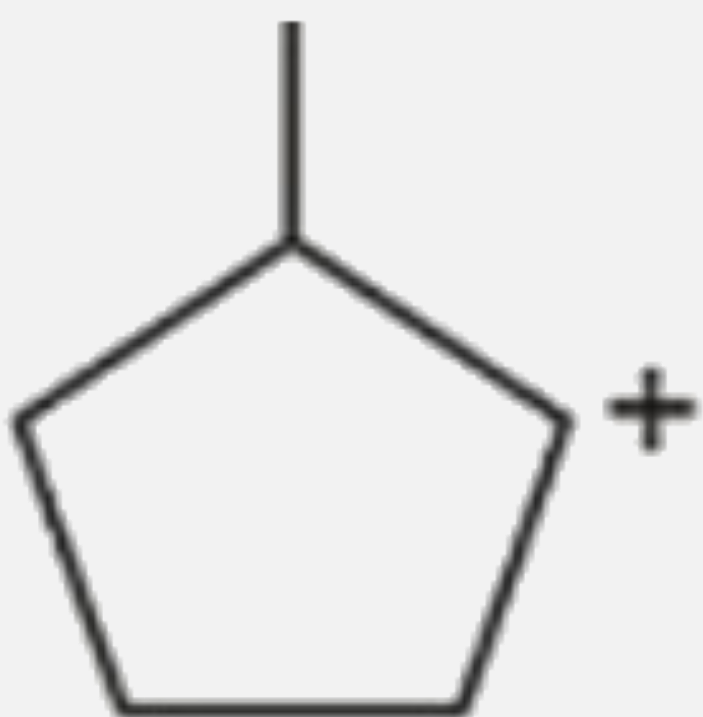
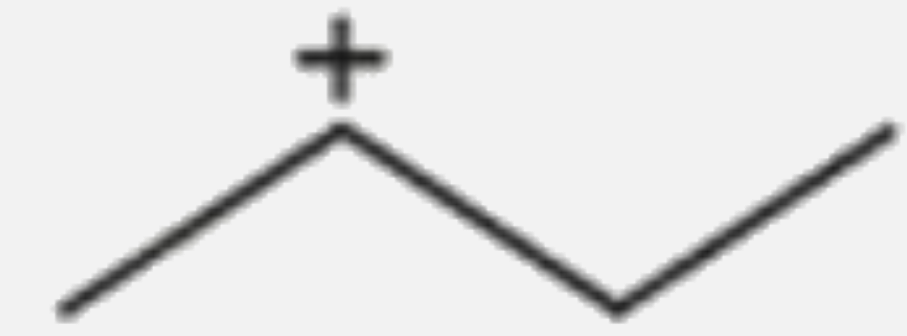
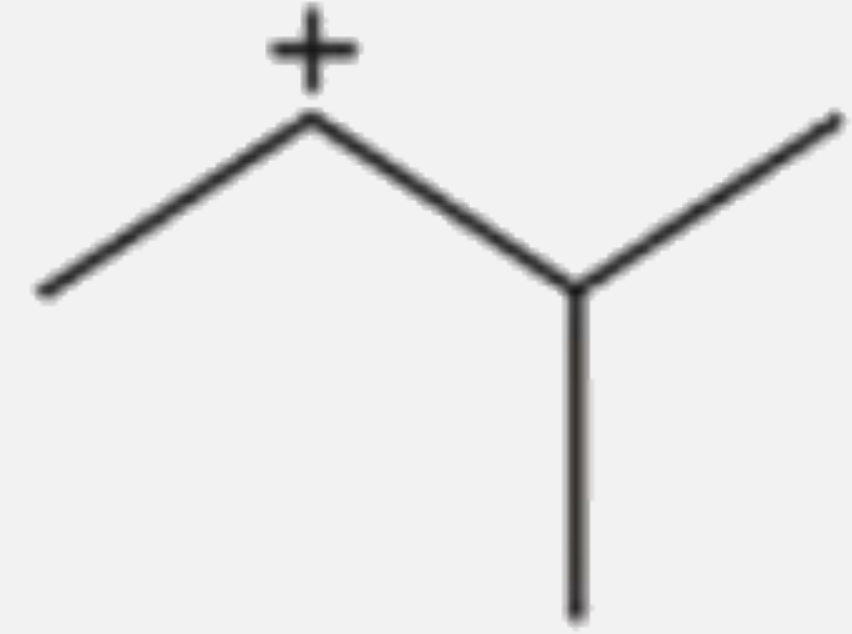
none of these
The correct Answer is:B
To determine which carbocation would not likely rearrange to a more stable carbocation, we need to analyze the stability of different types of carbocations (primary, secondary, and tertiary) and their potential for rearrangement.
1. Understand Carbocation Stability:
- Tertiary carbocations (3°) are the most stable due to hyperconjugation and inductive effects from surrounding alkyl groups.
- Secondary carbocations (2°) are less stable than tertiary but more stable than primary (1°).
- Primary carbocations (1°) are the least stable and are less likely to rearrange to a more stable form.
Hint: Remember the order of stability: Tertiary > Secondary > Primary.
2. Identify the Carbocations:
- Analyze the given carbocations in the question. Identify whether they are primary, secondary, or tertiary.
Hint: Look for the number of carbon atoms attached to the positively charged carbon atom.
3. Evaluate Rearrangement Possibilities:
- A primary carbocation can rearrange to a secondary carbocation if there is a possibility of a hydride or alkyl shift.
- A secondary carbocation can rearrange to a tertiary carbocation, which is more stable.
- A tertiary carbocation is already the most stable and typically does not rearrange.
Hint: Consider the possibility of shifts (hydride or alkyl) that would lead to a more stable carbocation.
4. Determine Which Carbocation Does Not Rearrange:
- If a carbocation is tertiary, it will not rearrange because it is already in its most stable form.
- If a carbocation is primary and cannot rearrange to a more stable form, it is also a candidate.
Hint: Focus on the carbocation that is either tertiary or a primary carbocation that lacks the ability to rearrange.
5. Select the Correct Option:
- Based on the analysis, identify which option corresponds to a carbocation that would not likely rearrange to a more stable carbocation.
Hint: Compare the stability of the carbocations and their potential for rearrangement based on the previous steps.
Conclusion:
After analyzing the stability and potential rearrangements of the carbocations, the answer to the question is Option B, as it represents a carbocation that would not likely rearrange to a more stable carbocation.
- Tertiary carbocations (3°) are the most stable due to hyperconjugation and inductive effects from surrounding alkyl groups.
- Secondary carbocations (2°) are less stable than tertiary but more stable than primary (1°).
- Primary carbocations (1°) are the least stable and are less likely to rearrange to a more stable form.
- Analyze the given carbocations in the question. Identify whether they are primary, secondary, or tertiary.
- A primary carbocation can rearrange to a secondary carbocation if there is a possibility of a hydride or alkyl shift.
- A secondary carbocation can rearrange to a tertiary carbocation, which is more stable.
- A tertiary carbocation is already the most stable and typically does not rearrange.
- If a carbocation is tertiary, it will not rearrange because it is already in its most stable form.
- If a carbocation is primary and cannot rearrange to a more stable form, it is also a candidate.
- Based on the analysis, identify which option corresponds to a carbocation that would not likely rearrange to a more stable carbocation.
After analyzing the stability and potential rearrangements of the carbocations, the answer to the question is Option B, as it represents a carbocation that would not likely rearrange to a more stable carbocation.
Similar Questions
Knowledge Check
Which of the folllowing carbocations would you expect to rearrange?
A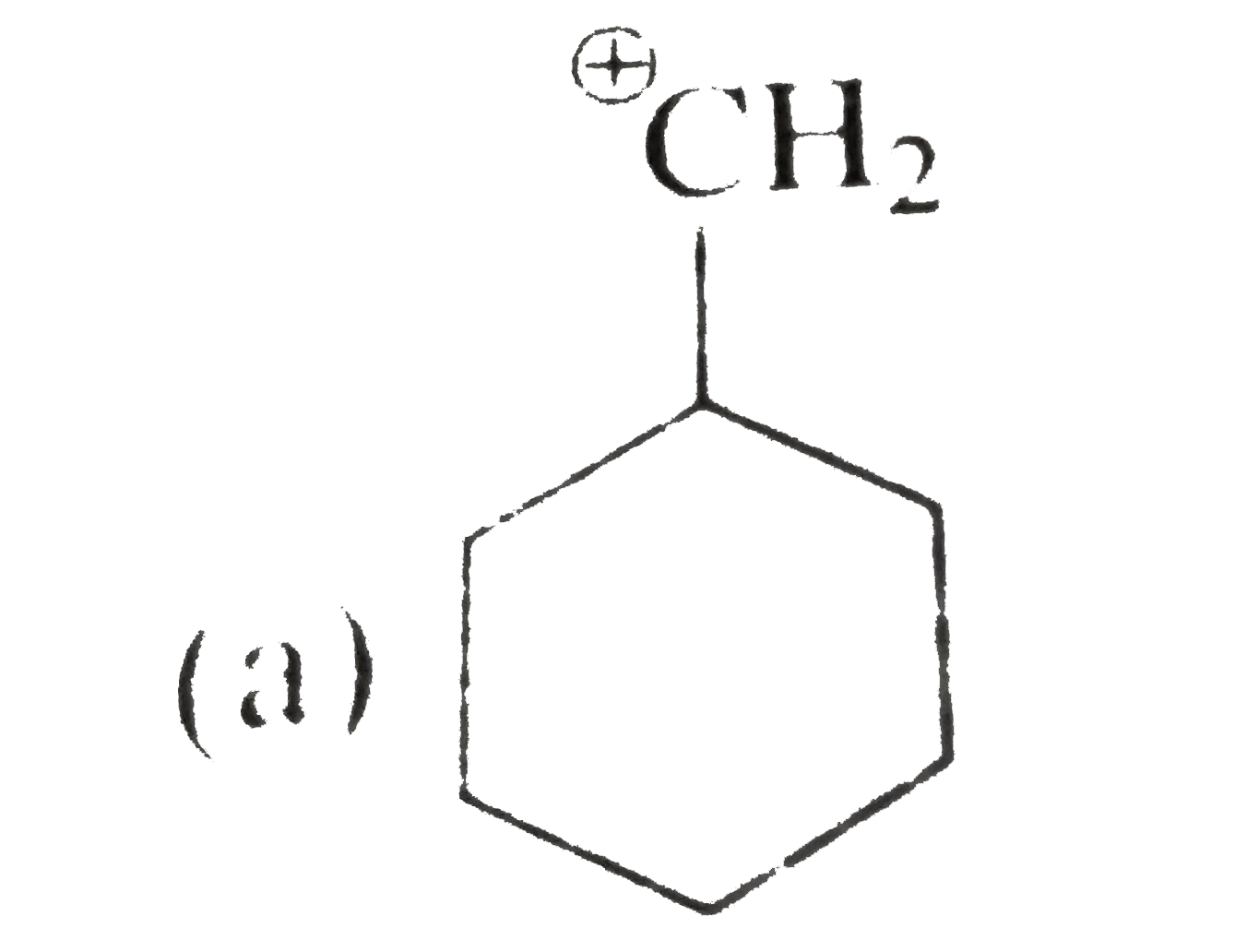 B
B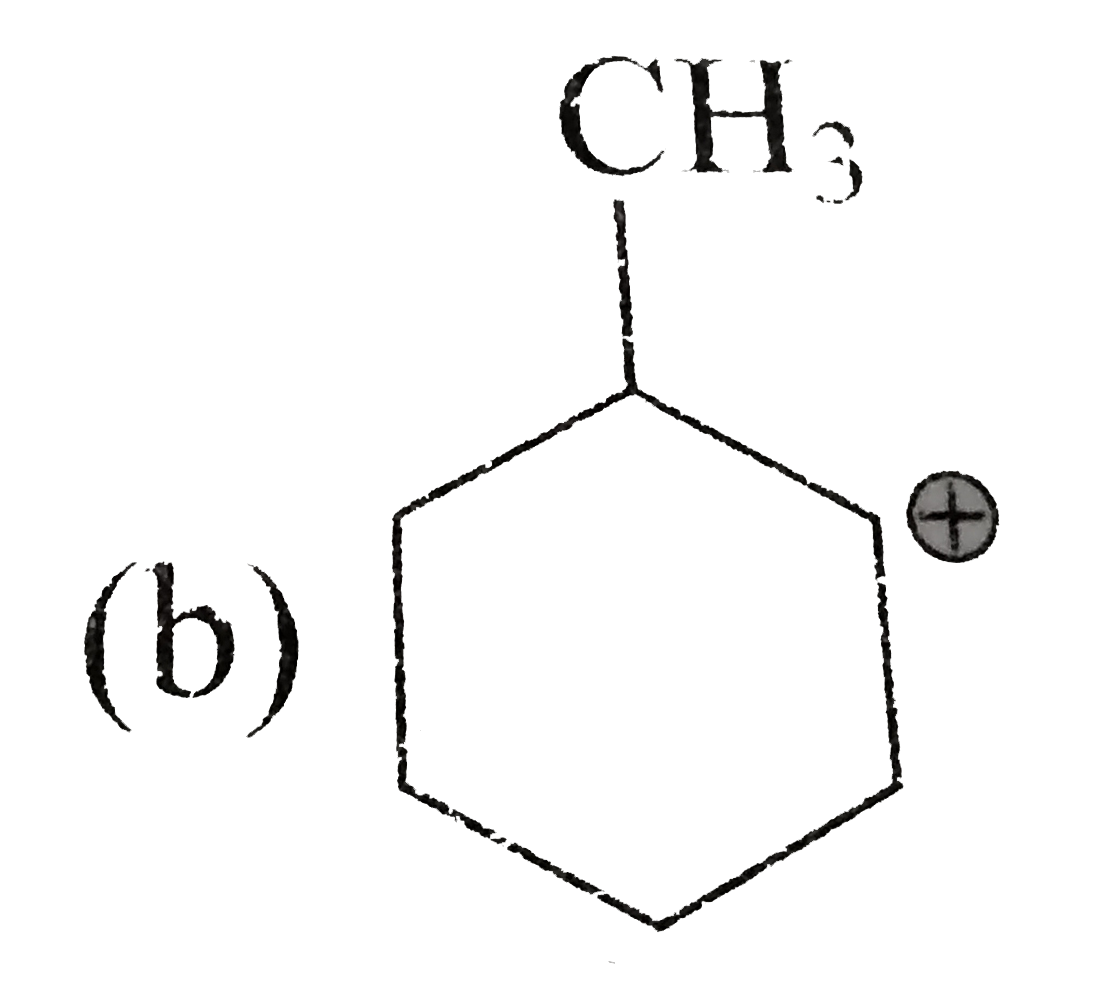 C
C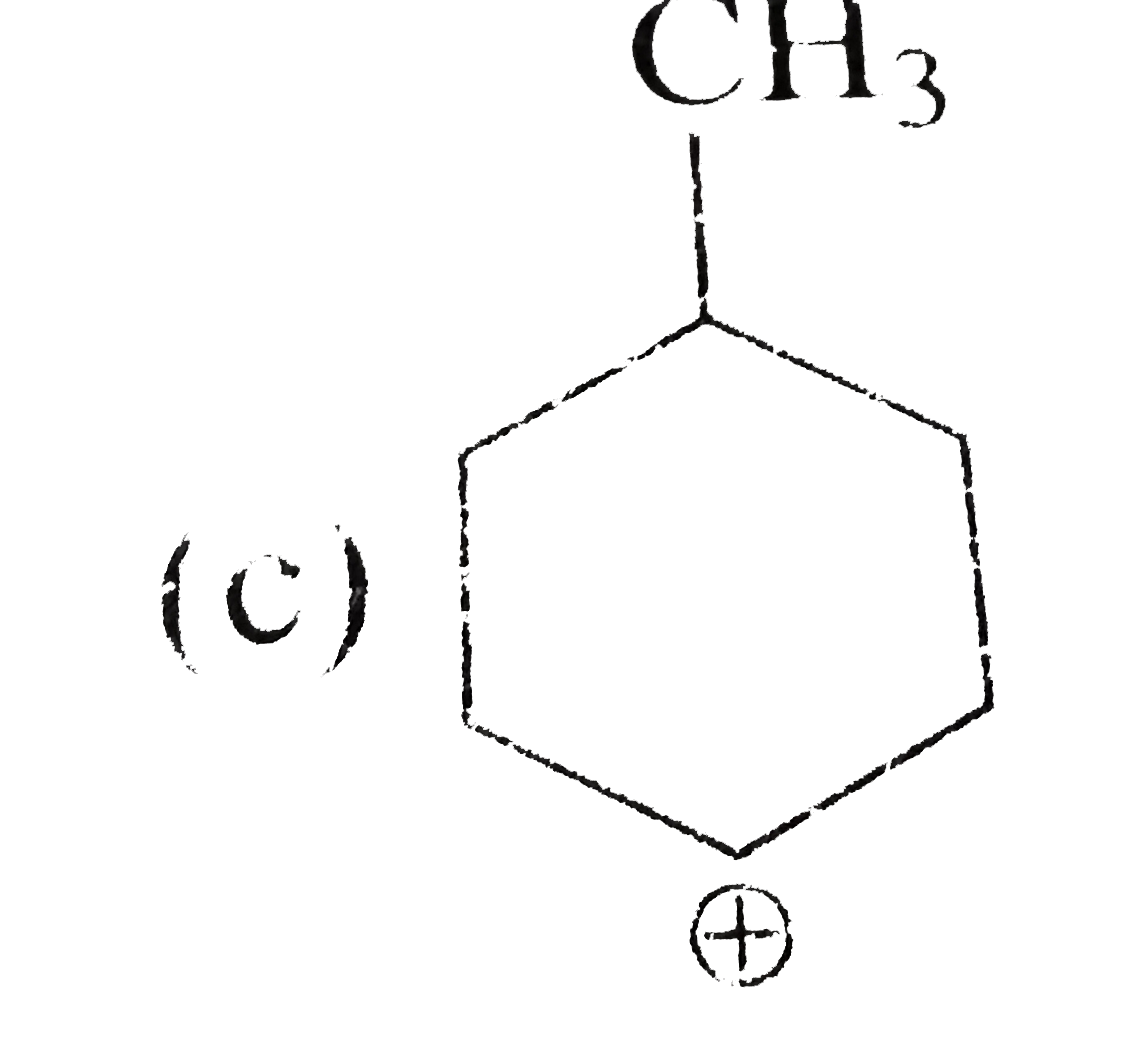 D
D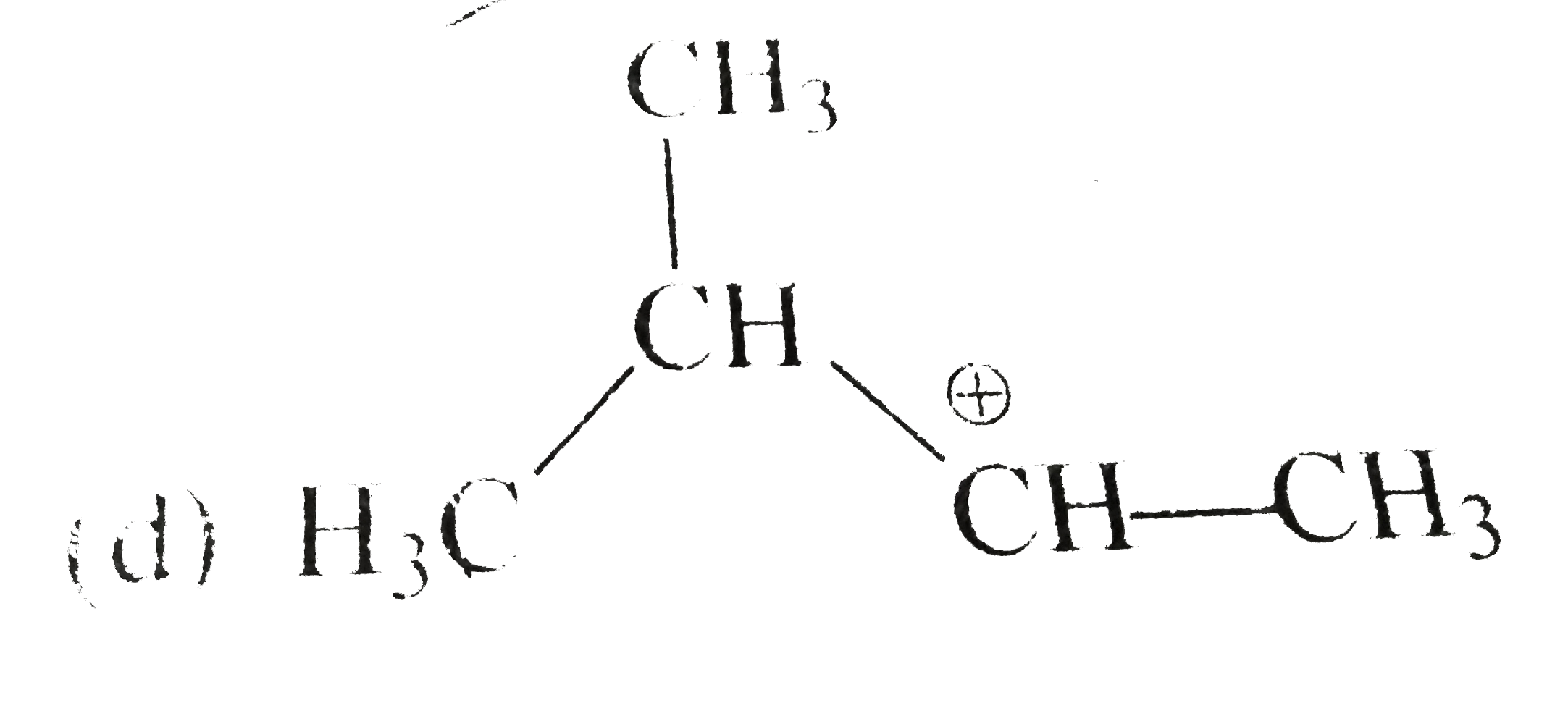
Which of the following carbocation is most stable?
APh3C+B(CH3)2C+HCCH3CH+2DCH2=CH−CH⊕2Which of the following carbocation is most stable?
A(CH3)3C⊕CH2B(CH3)3⊕CCCH3CH2⊕CH2DCH3⊕CHCH2CH3
Which of the following carbocations is most stable?
View SolutionWhich of the following carbocation is least stable
View SolutionWhich of the following carbocations is most stable ?
View SolutionWhich of the following carbocations is most stable ?
View SolutionWhich of the following carbocation is most stable ?
View Solution