Which of the following shapes of SF4 is more stable and why ?
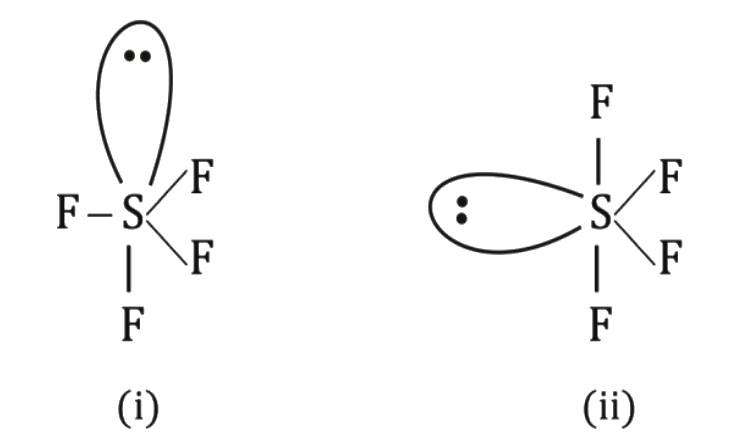
A
(i) , due to 3 lp - bp repulsion at 90∘
B
(ii), due to 2 lp - bp repulsion
C
Both are equally stable due to 2 lp - bp repulsions
D
Both are unstable since SF4 has tetrahedral shape
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video & image solution for Which of the following shapes of SF_4 is more stable and why ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Which of the following shapes of SF4 is more stable and why?
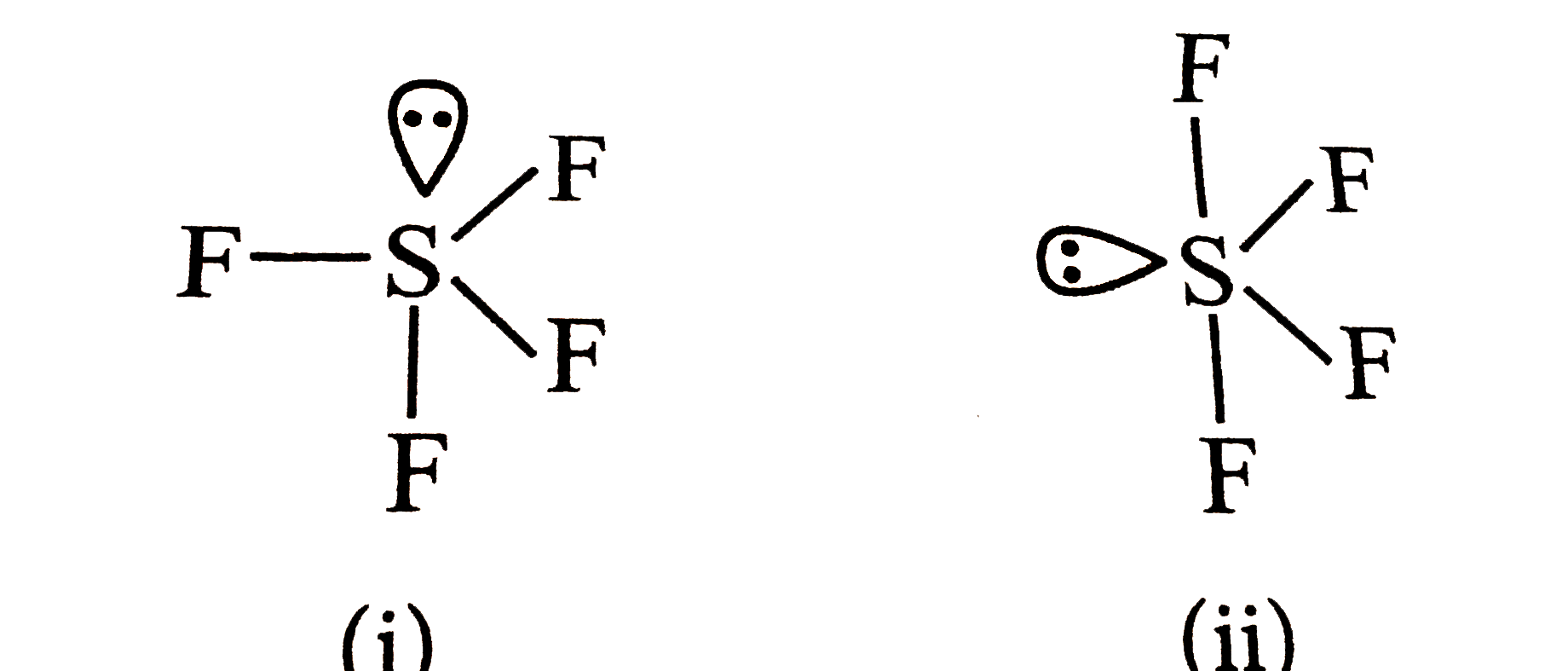
View SolutionMolecular shape of SF4,CF4 and XeF4 are
View SolutionThe molecular shapes of SF4,CF4andXeF4 are
View SolutionWhich of the following carbocation is more stable and why?
View SolutionWhich one is more stable and why?
View SolutionMolecular shapes of SF4,CF4 and XeF4 are
View SolutionThe shape of SF4 is
View SolutionDiscuss the shape of SF4?
View Solution

