If hydrogen and oxygen are mixed and kept in the same vessel at room temperature, the reaction does not take place to form water because
activation energy for the reaction is very high at room temperature
molecules have no proper orientation to react to form water
the frequency of collisions is not high enough for the reaction to take place
no catalyst is present in the reaction mixture
The correct Answer is:A
Step by step video, text & image solution for If hydrogen and oxygen are mixed and kept in the same vessel at room temperature, the reaction does not take place to form water because by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
Haloform reaction does not take place with
AacetoneB2-chloropropaneCethanolDmethanolHaloform reaction does not take place with :
AMethanolBEthanolCAcetoneD2 - Chloropropane.Cannizzaro reaction does not take place with
A(CH3)3CCHOB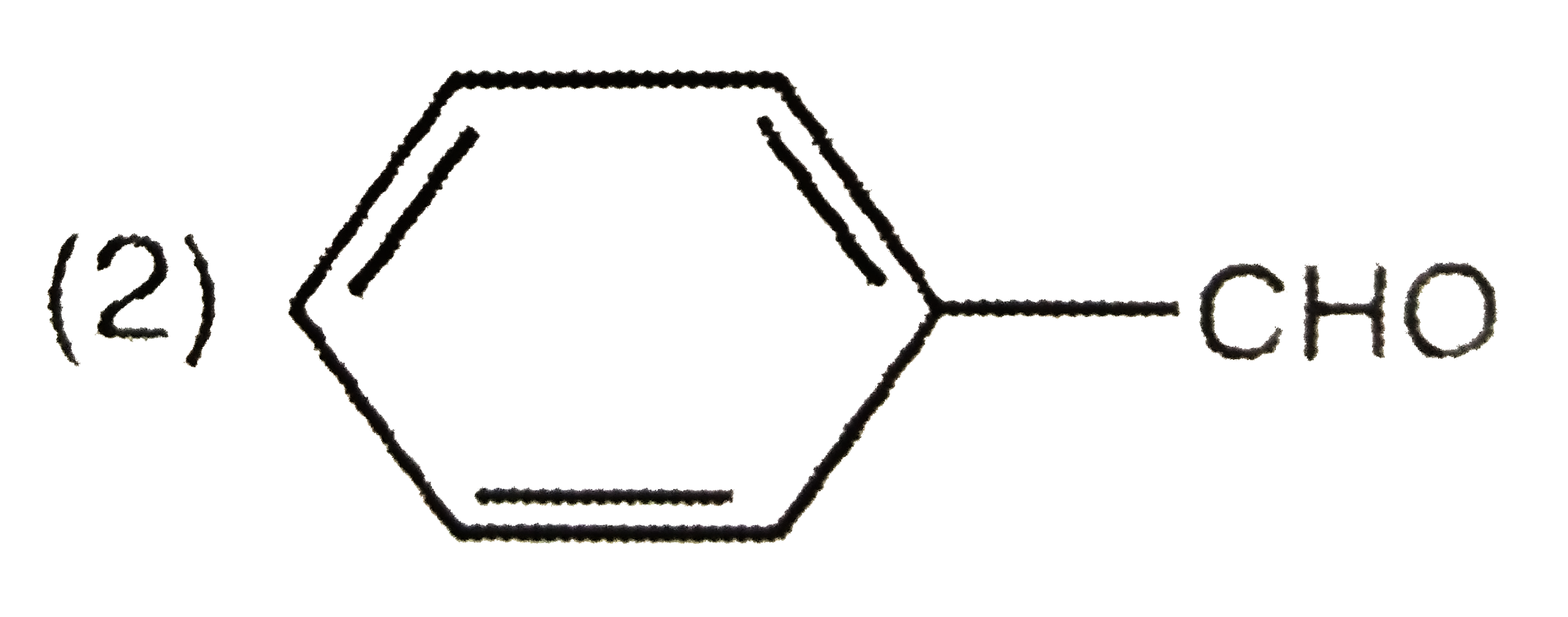 C
C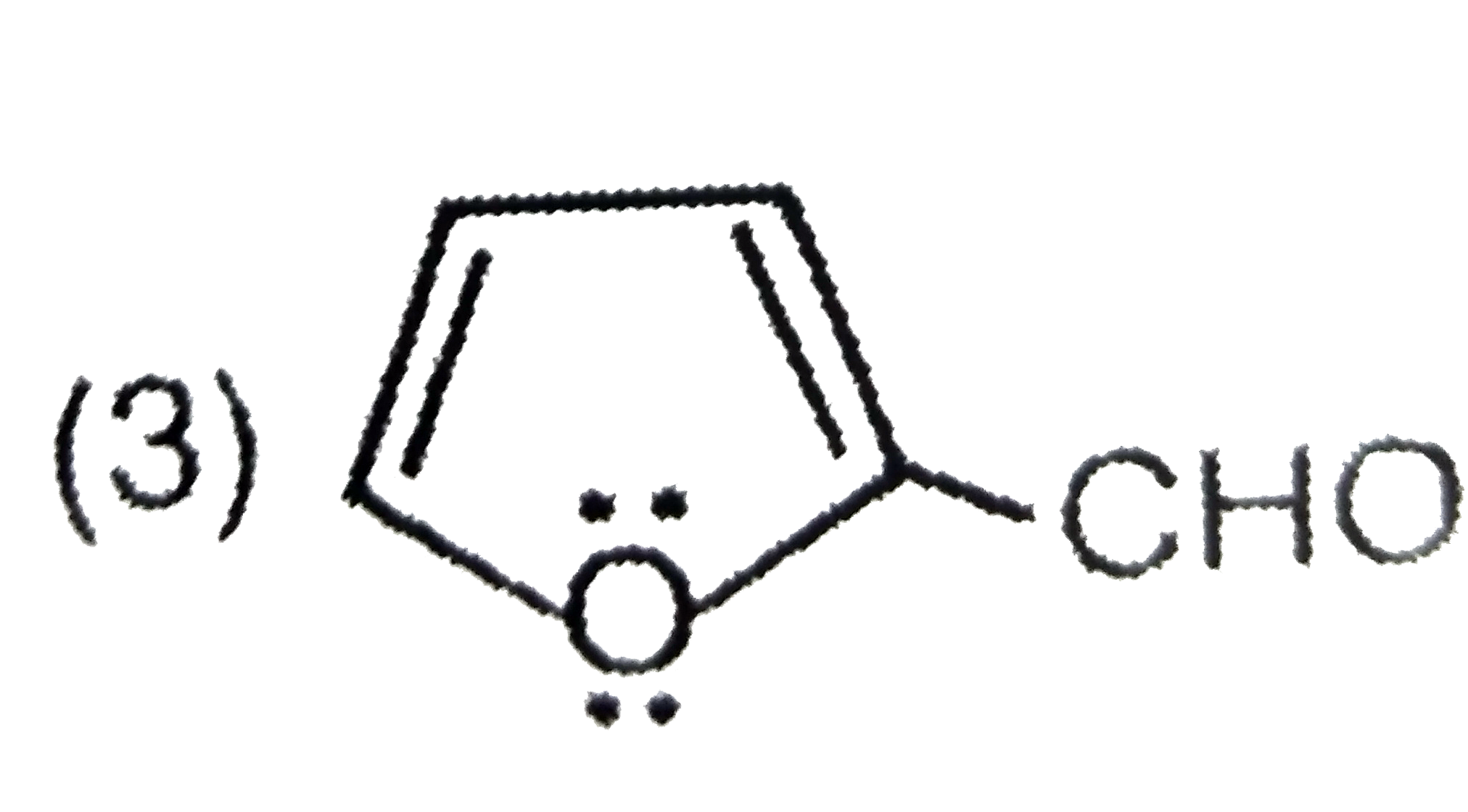 DCH3CHO
DCH3CHO
Identify the reaction that does not take place in blast furnace
View SolutionWhich of the following reaction does not take place
View SolutionWhich of the following reaction does not take place?
View SolutionIdentify the reaction that does not take place in a blast furnace
View SolutionWhich out of the following reactions does not take place ?
View Solution
