Phenol on reaction with H2SO4 at high temperature gives mainly
o - phenol sulfonic acid
para - phenol sulfonic acid
meta - phenol sulfonic acid
none of these
The correct Answer is:B
To solve the question regarding the reaction of phenol with H2SO4 at high temperature, we can follow these steps:
1. Identify the Reactants:
- The reactant is phenol (C6H5OH) and it is being reacted with sulfuric acid (H2SO4).
2. Understand the Nature of Phenol:
- Phenol is an activated aromatic compound. The hydroxyl group (-OH) is an electron-donating group, which increases the electron density on the benzene ring. This makes the ring more reactive towards electrophilic substitution reactions.
3. Electrophilic Substitution Reaction:
- When phenol reacts with sulfuric acid, the electrophile generated is the sulfonium ion (SO3H+). The reaction is an electrophilic aromatic substitution where the electrophile (SO3H) will attach to the benzene ring.
4. Determine the Positions for Electrophilic Attack:
- Due to the resonance structures of phenol, the electron density is increased at the ortho and para positions of the benzene ring. Therefore, the electrophile can attack either the ortho or para position, but not the meta position.
5. Consider the Reaction Conditions:
- The reaction is conducted at high temperatures. At elevated temperatures, steric hindrance and stability of the product become significant factors. The ortho product (ortho phenol sulfonic acid) experiences steric repulsion due to the bulky SO3H group being close to the -OH group.
6. Identify the Major Product:
- Although both ortho and para phenol sulfonic acids can be formed, the para product is more stable due to less steric hindrance. Therefore, at high temperatures, the major product will be para phenol sulfonic acid.
7. Conclusion:
- The main product formed when phenol reacts with H2SO4 at high temperature is para phenol sulfonic acid.
Final Answer:
The main product is para phenol sulfonic acid.
---
- The reactant is phenol (C6H5OH) and it is being reacted with sulfuric acid (H2SO4).
- Phenol is an activated aromatic compound. The hydroxyl group (-OH) is an electron-donating group, which increases the electron density on the benzene ring. This makes the ring more reactive towards electrophilic substitution reactions.
- When phenol reacts with sulfuric acid, the electrophile generated is the sulfonium ion (SO3H+). The reaction is an electrophilic aromatic substitution where the electrophile (SO3H) will attach to the benzene ring.
- Due to the resonance structures of phenol, the electron density is increased at the ortho and para positions of the benzene ring. Therefore, the electrophile can attack either the ortho or para position, but not the meta position.
- The reaction is conducted at high temperatures. At elevated temperatures, steric hindrance and stability of the product become significant factors. The ortho product (ortho phenol sulfonic acid) experiences steric repulsion due to the bulky SO3H group being close to the -OH group.
- Although both ortho and para phenol sulfonic acids can be formed, the para product is more stable due to less steric hindrance. Therefore, at high temperatures, the major product will be para phenol sulfonic acid.
- The main product formed when phenol reacts with H2SO4 at high temperature is para phenol sulfonic acid.
The main product is para phenol sulfonic acid.
Similar Questions
Knowledge Check
Phenol is heated with conc. H2SO4 at high temperature gives,
Ao-phenol sulphonic acidBp-phenol sulphonic acidCm-phenol sulphonic acidDall of thesePhenol reacts with bromine in H2O at high temperature to give
Am-bromophenolBo and p-bromophenolCp-bromophenolD2,4,6-tribromophenolPhenol reacts with Br2 in CCl4 at low temperature to give
Ao- and p- bromophenolBm-bromophenolCp-bromophenolD2, 4, 6-tribromophenol
The reaction
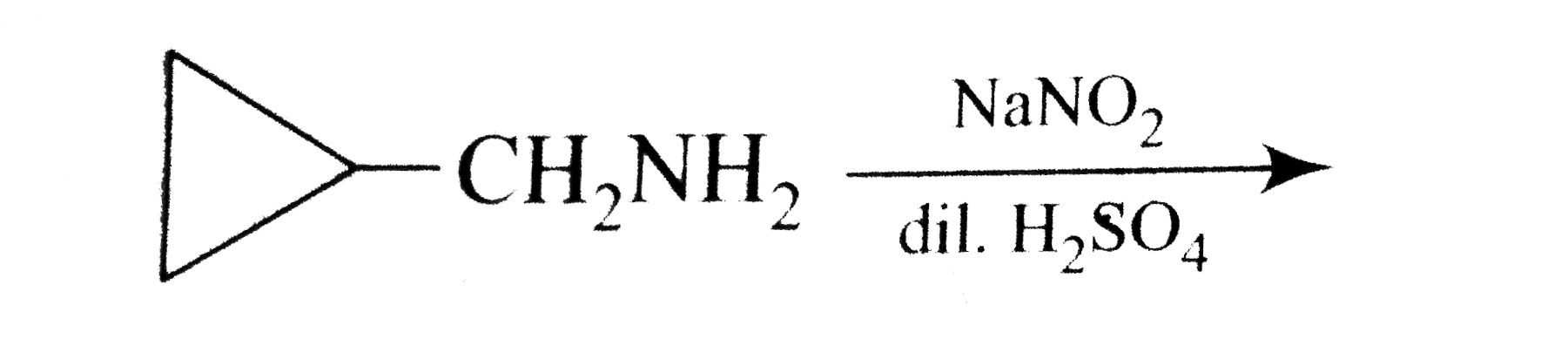
gives mainly
View SolutionPhenol reacts with Br2 in CCl4 at low temperature to give
View SolutionPhenol reacts with bromine in CS2 at low temperature to give
View SolutionWhen concentrated H2SO4 at room temperature , phenol gives
View SolutionPhenol on treatment with conc. H2SO4 at 300 K gives
View Solution

