Which of the following metals are obtained by electrolysis of their chlorides in molten state?
(i)Na
(ii)Ca
(iii)Fe
(iv)Cu
A
I and iv
B
iii and iv
C
I and iii
D
I and ii
Video Solution
Text Solution
The correct Answer is:D
Sodium (Na) and caicium (Ca) being highly reactive are obtained by electrolysis of their chlorides in molten state.
|
Similar Questions
Knowledge Check
Which of the following metals are extracted by the electrolysis of their molten chlorides ?
ANaandHgBHgandMgCNaandMgDCuandFeMetals are refined by using different methods. Which of the following metals are not refined by electrolytic refining?
(i) Au
(ii) Cu
(iii)Na
(iv) KA(i)and (ii)B(i) and (iii)C(ii) and (iii)D(iii) and (iv)Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining?
(i) Ag (ii) Cu
(iii) Na (iv) MgA(i) and (ii)B(i) and (iii)C(ii) and (iii)D(iii) and (iv)
Out of the following the number of metals that can not be obtained by electrolysis of the aqueous solution of their sals are
Ag,Mg,Cu,Al,Au,Ca,Na
View SolutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
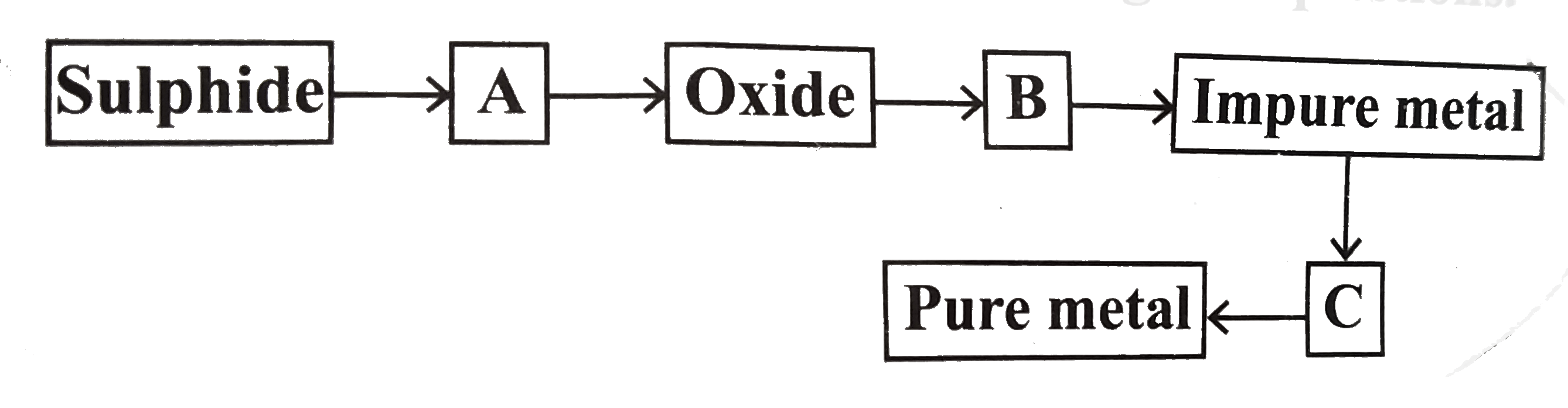
(iv) Some of the following metals are obtained by electrolysis of their fused salts : Al,Na,Cu,Ag,Mg,Ca. These metals are :
View SolutionWhich of the following are not state functions of state variables?
(i) Internal energy
(ii) Heat
(iii) Work
(iv) Volume
View SolutionWhich of the following metals continue to burn in the atmosphere of CO2 ?
(i) Na
(ii) K
(iii) Mg
(iv) Ca
View SolutionThe process of extracting metals by electrolysis of their oxides, hydroxides or chlorides in the fused state is called
View Solution
