An element E has a valency of 4.
(i) What will be the formula of its chloride ?
(ii) What will be the formula of its sulphide ?
In order to solve this problem, we should know the valencis of chlorine and sulphur elements (because chlorine forms the choride and sulphur forms the sulphide). Knowing that the valency of chlorine is 1 and that of sulphur is 2, the abvoe problem can be solved as follows :
(i) Formula of chloride of element E
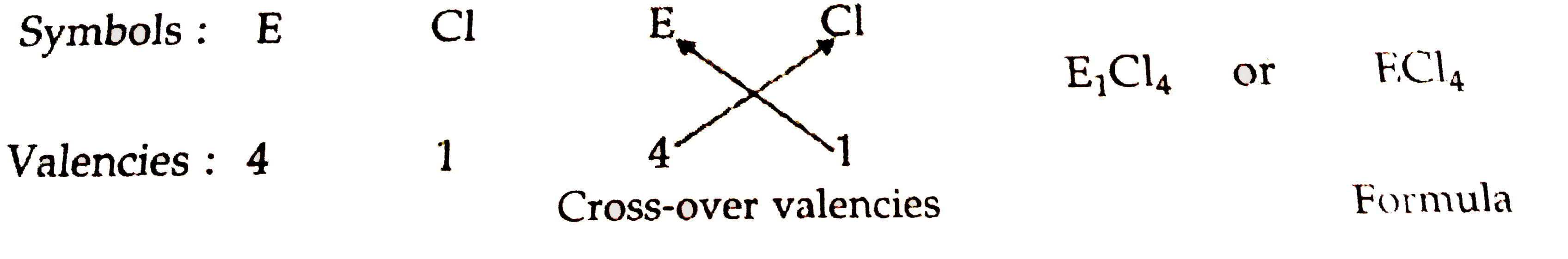
Thus, the formula of chloride of element E is ECl4.
(ii) Formula of sulphide of element E
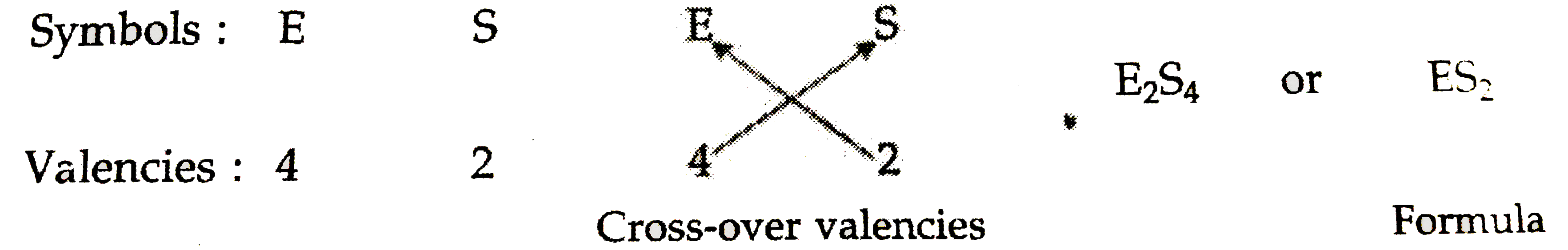
Thus, the formula of sulphide of element E is ES2.
(It should be noted that the element E having a valency of 4 is like carbon, C. So, the chloride ECl4 is like carbon tetrachloride, CCl4, and the sulphide, ES2 is like carbon disulphide, CS2).
Similar Questions
An element X is in group of the periodic table:
(a) What will be the formula of its chloride?
(b)What will be the formula of its oxide?
View SolutionAn element X is in group 13 of the periodic table.
(a) what will be the formula of its chloride ?
(b) What will be the formula of its oxide ?
View Solution
Knowledge Check
An element 'P' has atomic number 56. What will be the formula of its halide?
APXBPX2CPX3DP2X3The formula of a metal chloride is MCl3. The formula of its phosphate is
AM2PO4BM3PO4CM(PO4)2DMPO4An element X has atomic number 19.What will be the formula of its oxide?
AX2OBXOCXO2DX2O3
The formula of the chloride of a metal is MCl2. What will be the formula of its sulphate.
View SolutionAn element Z has a valency of 3. What is the formula of oxide of Z ?
View SolutionAn element Z has a valency of 3. What is the formula of its oxide?
View SolutionThe formula of the chloride of a metal is MCl2. What will be the formula of its sulphate.
View SolutionThe formula of carbonate of a metal M is M2CO3.
(a) What will be the formula of its iodide ?
(b) What will be the formula of its nitride ?
(c) What will be the formula of its phosphate ?
View Solution
