Aqeuous solutions of zinc sulphae and iron sulphate were taken in test tubes I and II by four students A,B, C and D. Metal pieces of iron and zinc were dropped in the two solutions and observations made after several hours and recorded in the form of a table as given below:
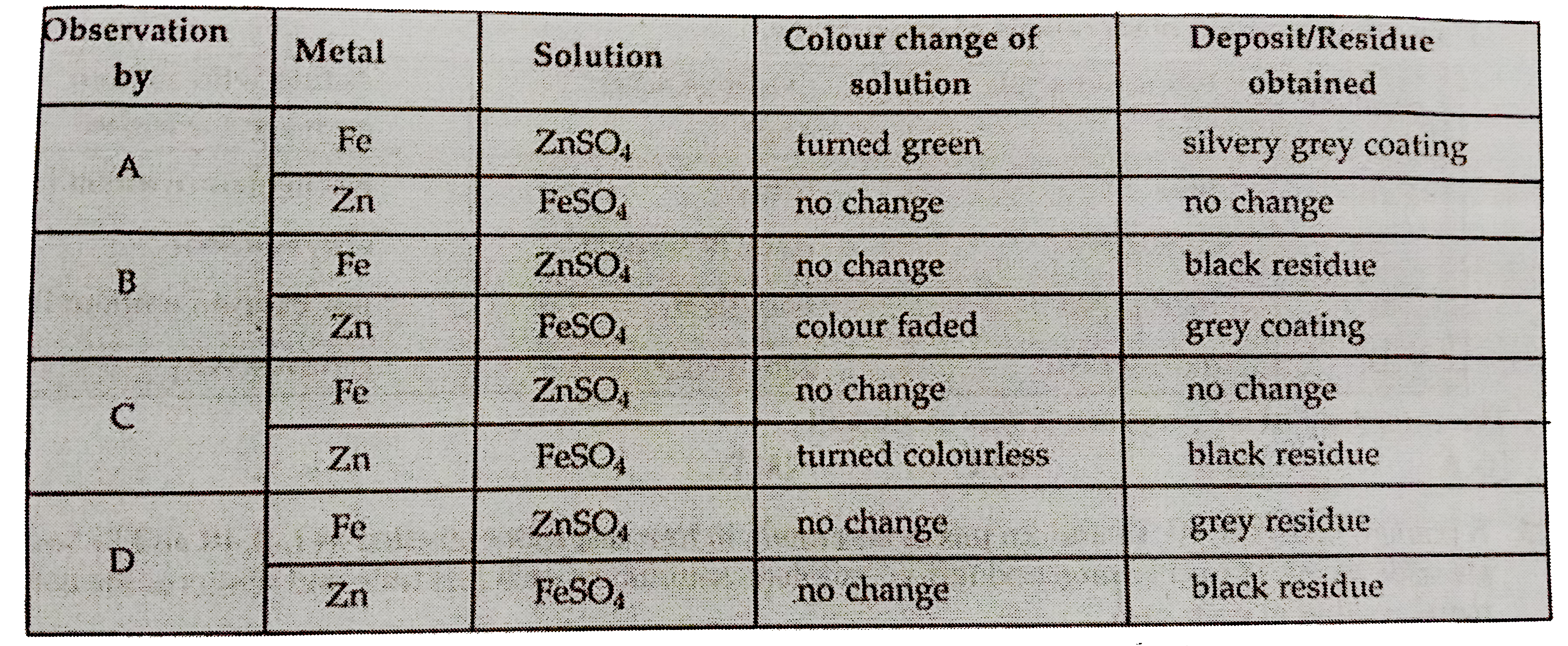
The correct reporting has been made in observation reported by the student:
A
B
C
D
The correct Answer is:A
NA
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - LAKHMIR SINGH & MANJIT KAURCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - LONG ANSWER TYPE
3 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - LAKHMIR SINGH & MANJIT KAURCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - VERY SHORT ANSWER TYPE
6 Videos
- TEST PAPER 1BOOK - LAKHMIR SINGH & MANJIT KAURCHAPTER - TEST PAPER 1EXERCISE - Section B
4 Videos
Similar Questions
(a) State Mendeleev's periodic law.
What chemical properties of elements were used by Mendeleev in creating his periodic table?
(c) State any three limitations of Mendeleev's classification of elements.
(d) Besides gallium, which two other elements have since been discovered for which Mendeleev had left gaps in the periodic table?
(e) Which group of elements was missing from Mendeleev's original periodic table?
View Solution(a) How does the chemical reactiviyt of alkali metals vary on going down in group 1 of the periodic table?
(b) How does the chemical reactivity of the halogens vary on going down in group 17 of the periodic table?
View Solution(a) A student dropped a few pieces of marble in dilute hydrochloric acid contained in
a test tube. The evolved gas was passed through lime water. What change would
be observed in lime water ? Write balanced chemical equations for both the
changes observed.
(b) State the chemical property in each case on which the following uses of baking
soda are based:
(i) as an antacid (ii) as a constituent of baking powder.
View SolutionFour students I, II, III and IV were asked to examine the changes for blue and red litmus paper strips with dilute HCI (solution A) and dilute NaOH (solution B). The following observations were reported by the four students. The sign (-) indicating no colour change.
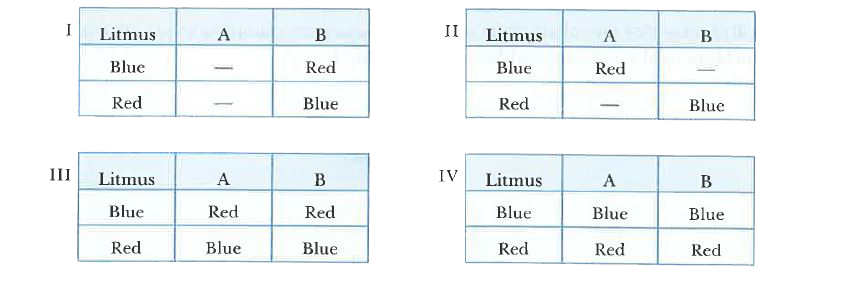
Which student gave the correct observation?
View SolutionFour students A,B,C and D observed the chemical properties of acetic acid and recorded their observations in the table given below. Which student made the correct observation?
View SolutionFour students A, B, C, and D took aqueous solutions of zinc sulphate and iron sulphate in two test tubes I and II and dropped metal pieces of Iron and Zinc in the two solutions. After several hours, they made observations and recorded their findings in the form of a table given below:
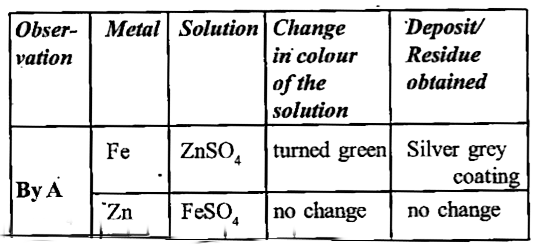
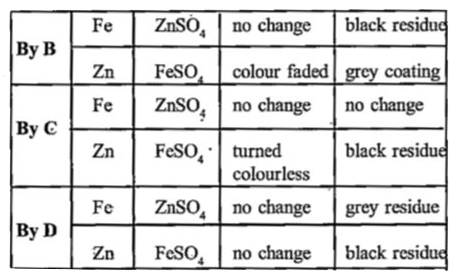
Who reported the correct observation?
View SolutionZinc pieces were placed in each of the four test tubes containing different salt solutions as shown below: In which cases will colour change be observed in solutions?
View SolutionThree test tubes marked A, B and C are taken. In test tube A copper sulphate solution and iron are added. In test tube B, copper sulphate solution and zinc are added. In test tube C, iron sulphate solution and copper are added. In which test tube will the experimenter observe change and why?
View Solution
