Among the following, the species that is both tetrahedral and diamagnetic is-
A
[NiCl4]2−
B
[Ni(CN)4]2−
C
Ni(CO)4
D
[Ni(H2O)6]2+
Video Solution
Text Solution
The correct Answer is:C
[Ni(CO)4]
Ni∘=3d84s2
CO=SFL,Ni∘=3d104s∘
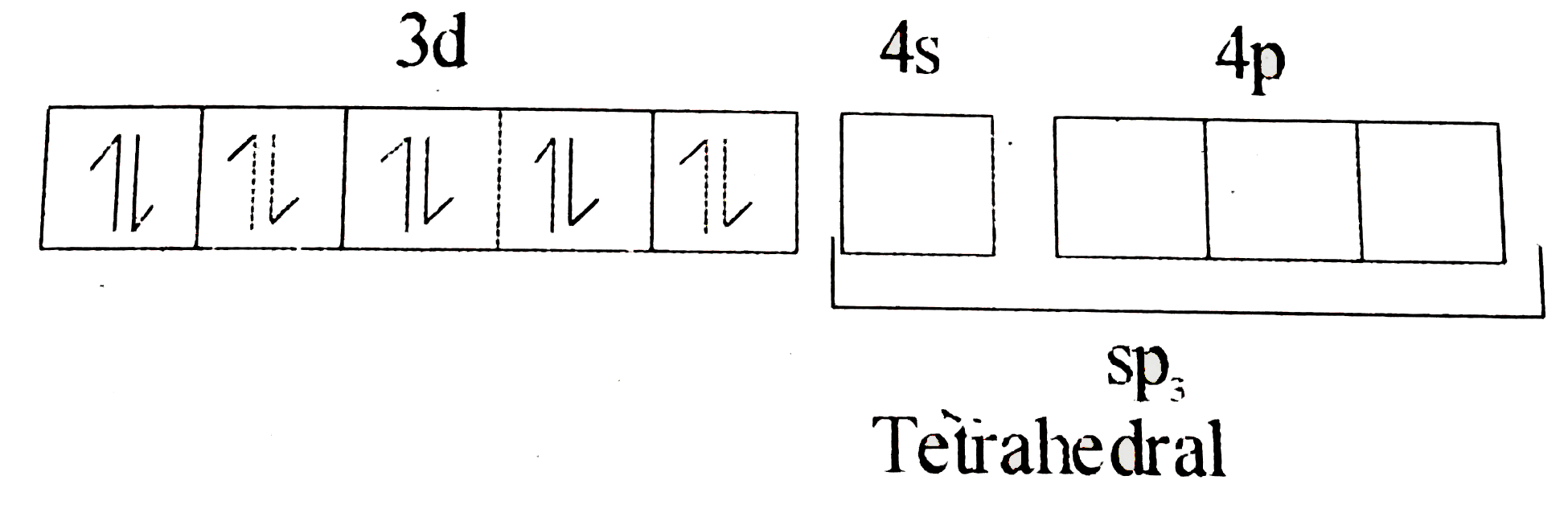
Unpaired electron = 0, Diamagnetic
|
Similar Questions
Knowledge Check
Among the following, the species that is both paramagnetic and coloured is :
A[MnO4]2−BK4[V(CN)6]C[VO4]3−DCrO2Cl2Among the following, the species with the highest bond order is-
AO2BF2CO+2DF2Among the following, the species with identical bond order are
ACO and O2−2BO−2 and COCO2−2 and B2DCO and N+2
Among the following, the species having the smallest bond is
View SolutionThe species having tetrahedral shape is
View SolutionThe species having tetrahedral shape is
View SolutionWhich of the following chemical species is tetrahedral, diamagnetic as well as coloured ?
View SolutionWhich of the following chemical species is tetrahedral, diamagnetic as well as coloured ?
View Solution

