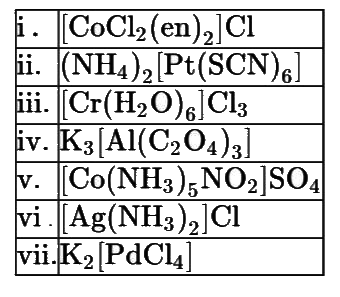
The complex that can exhibit linkage isomerism is
A
[Co(NH3)5(H2O)]Cl3
B
[Co(NH3)5(NO2)]Cl2
C
[Co(NH3)5(NO3)](NO3)2
D
[Co(NH3)5Cl]SO4
Video Solution
Text Solution
The correct Answer is:B
−NO2 is ambidentate ligand as it connect with metal by N or O atom.
|
Similar Questions
Knowledge Check
The complex that exhibits Co-ordination isomerism is
A[Cr(NCS)(H2O)5]2+B[Cr(NH3)6]Cl3C[Cr(NH3)6][Co(CN)6]D[CoCl2(NH3)4]Cl.H2OThe complex that can show optical isomerism is:
Acis−[CrCl2(ox)2]−3Btrans−[CrCl2(ox)2]−3CBoth (A) & (B)DNeither (A) and (B)The complex which exhibits cis-trans isomerism as well as can be resolved into d and ? forms:
A[Be(acac)2]B[Ir(H2O)3(NH3)3]3+C[Cr(en)3]3+D[Rh(H2O)2(en)2]3+
The complex show below can exhibit
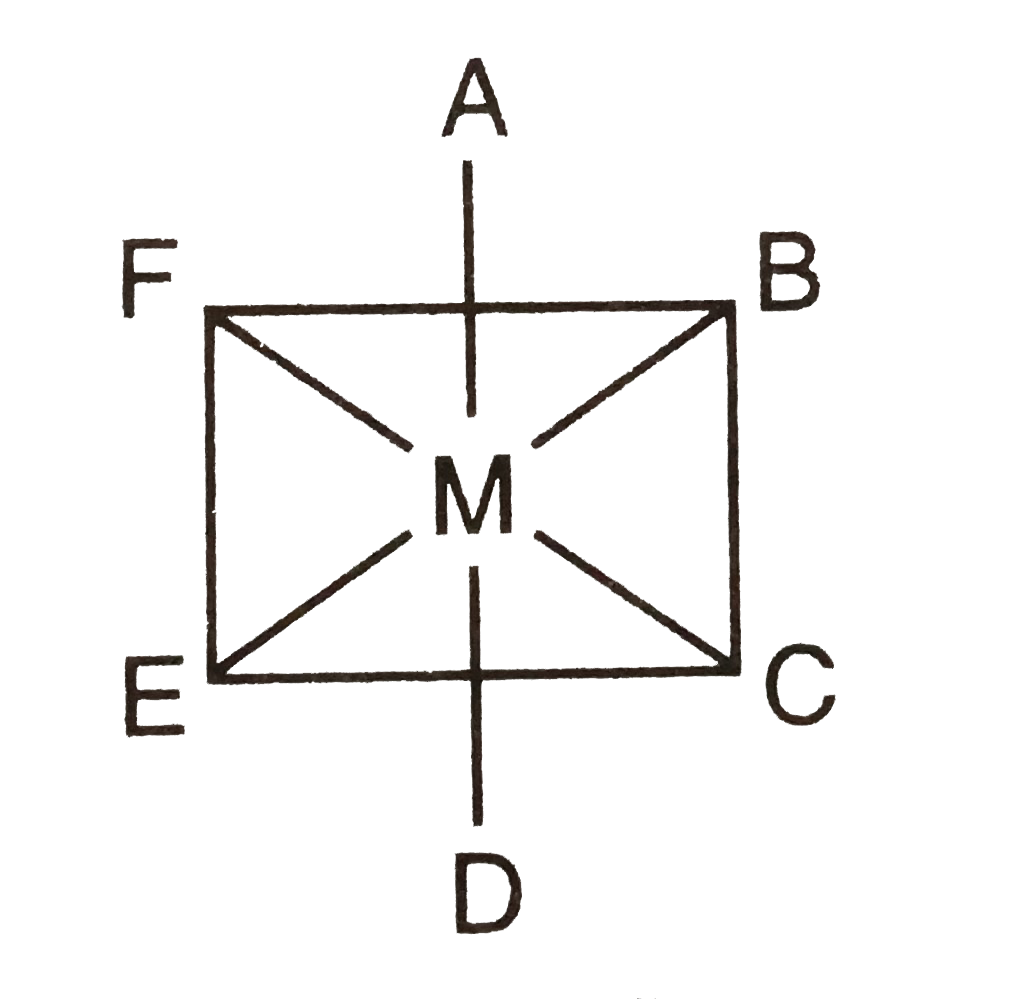
View SolutionThe complex shown below can exhibit
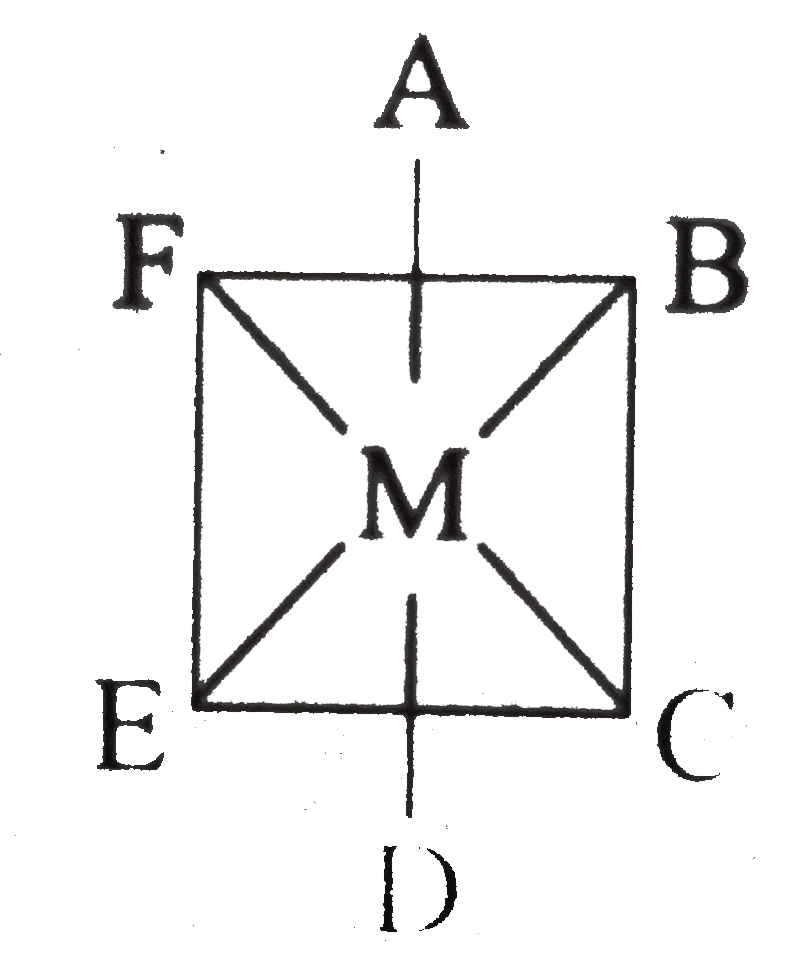
View SolutionWhich of the following complexes can show linkage isomerism?
View SolutionAssertion: All square planar complexes can exhibit geometrical isomerism
Reason: In square planar complexes .
View SolutionWhich of the following complexes can exhibit optical isomerism? (en=H2N−CH2−CH2−NH2)
View Solution