Which of the following relationships is/are not true ?
A
Most probable velocity =√2RTM
B
PV=32kT
C
Compressibility factor Z=PVnRT
D
Average kinetic energy of gas =12kT
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for Which of the following relationships is/are not true ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following relationship is/are not true ?
AMost prabable velocity ∝=√3RTmBPV=23kTCCompressibility factor Z=PVnRTDAverage kinetic energy of a gas =12kTWhich of the following relationships is true?
AMolecular mass =(Vapor density of gas)2BMolecular mass =12 (Vapor densiy of gas)CMolecular mass =2×(Vapor density of gas)DMolecular mass =√Vapor density of gasWhich of the following relationship is true?
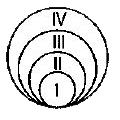 AI-Whole Numbers, II-Natural Numbers, III-Integers, IV-Rational NumbersBI-Rational Nurnbers, II-Integers, III-Whole Numbers, IV-Natual NumbersCI-Natural Numbers, II-Whole Numbers,
AI-Whole Numbers, II-Natural Numbers, III-Integers, IV-Rational NumbersBI-Rational Nurnbers, II-Integers, III-Whole Numbers, IV-Natual NumbersCI-Natural Numbers, II-Whole Numbers,
III-Integers, IV-Rational NumbersDNone of the above
Which of the following relationship are correct?
View SolutionWhich of the following relationship is correct ?
View SolutionWhich of the following relationship is correct ?
View SolutionWhich of the following relationship is correct ?
View SolutionCarbon, silicon and germanium atoms have four valence electrons each. Their valence and conduction bands are separated by energy band gaps represented by (Eg)C,(Eg)Si and (Eg)Ge, respectively. Which one of the following relationship is true in their case?
View Solution
