The essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO2. If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called the critical pressure (Pc). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows:
Vc=3b,Pc=a27b2,Tc=8a27Rb
Gas A and can be liquefied at room temperature by applying pressure but gas B cannot. This reflects:
critical temperature of B is less than that of A
critical temperature of B is greater than that of A
A and B have critical temperature greater than room temperature
critical temperature of both are equal
The correct Answer is:A
Step by step video, text & image solution for The essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO_(2). If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (T_(c)). The pressure required to liquefy a gas at this temperature is called the critical pressure (P_(c)). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows: V_(c) = 3b, P_(c) = (a)/(27b^(2)), T_(c) = (8a)/(27 Rb) Gas A and can be liquefied at room temperature by applying pressure but gas B cannot. This reflects: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STATES OF MATTER (GASES AND LIQUIDS)Book:OP TANDONChapter:STATES OF MATTER (GASES AND LIQUIDS)Exercise:p 4
Explore 6 Videos
- STATES OF MATTER (GASES AND LIQUIDS)Book:OP TANDONChapter:STATES OF MATTER (GASES AND LIQUIDS)Exercise:p 5
Explore 9 Videos
- STATES OF MATTER (GASES AND LIQUIDS)Book:OP TANDONChapter:STATES OF MATTER (GASES AND LIQUIDS)Exercise:p 2
Explore 7 Videos
- SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)Book:OP TANDONChapter:SOLUTIONS (GENERAL AND COLLIGATIVE PROPERTIES)Exercise:Self Assessment
Explore 31 Videos
- STATES OF MATTER (SOLID STATE)Book:OP TANDONChapter:STATES OF MATTER (SOLID STATE)Exercise:SELF ASSESSMENT Section VI
Explore 3 Videos
Similar Questions
Kinetic theory of gases is a generalization offered by Maxwell. Boltzmann, Clausius, etc. to explain the behaviour of ideal gases. This theory assumes that ideal gas molecules neither attract nor repel each other. Average kinetic energy of gas molecules is directly proportional to the absolute temperature. A gas equation called kinetic gas equation was derived on the basis of kinetic theory
PV=13mv2
GasDensityA0.82gL−1B0.26gL−1C0.51gL−1
Pick up the correct statement/statements
1. Gas A will tend to lie at the bottom
2. The number of atoms fo various gases A, B and C are same.
3. The gases will diffuse to form homogeneous mixture.
4. Average kinetic energy of each gas is same
View SolutionSurface tension of a liquid is a molecular phenomenon of liquids involving the force of cohension along the liquid molecules. It is scalar quantity and is numberically equal to the surface energy. Numerically, it is proved that the potential soluble salts and surfce active substance. Sparingly soluble salts and surface acitve substances decrease the surface tension of the liquid. However, the fairly soluble solutes increase the surface tension of the liquid. Surface tension of a liquid is independent of surface area but it depends on the intemolecular forces and the temperature.
Which among the following is not the unit of surface tension ?
View SolutionThe gas molecules randomly move in all directions and collide with each other and with the wall of the container. If is difficult to determine the speed of an individual molecule but it had become passible to work out the distribution of molecules among different molecular speeds. This is known as Maxwell Boltzmann distribution
consider the following graph about Maxwell's distribution of speed at two different temperature T1 and T2
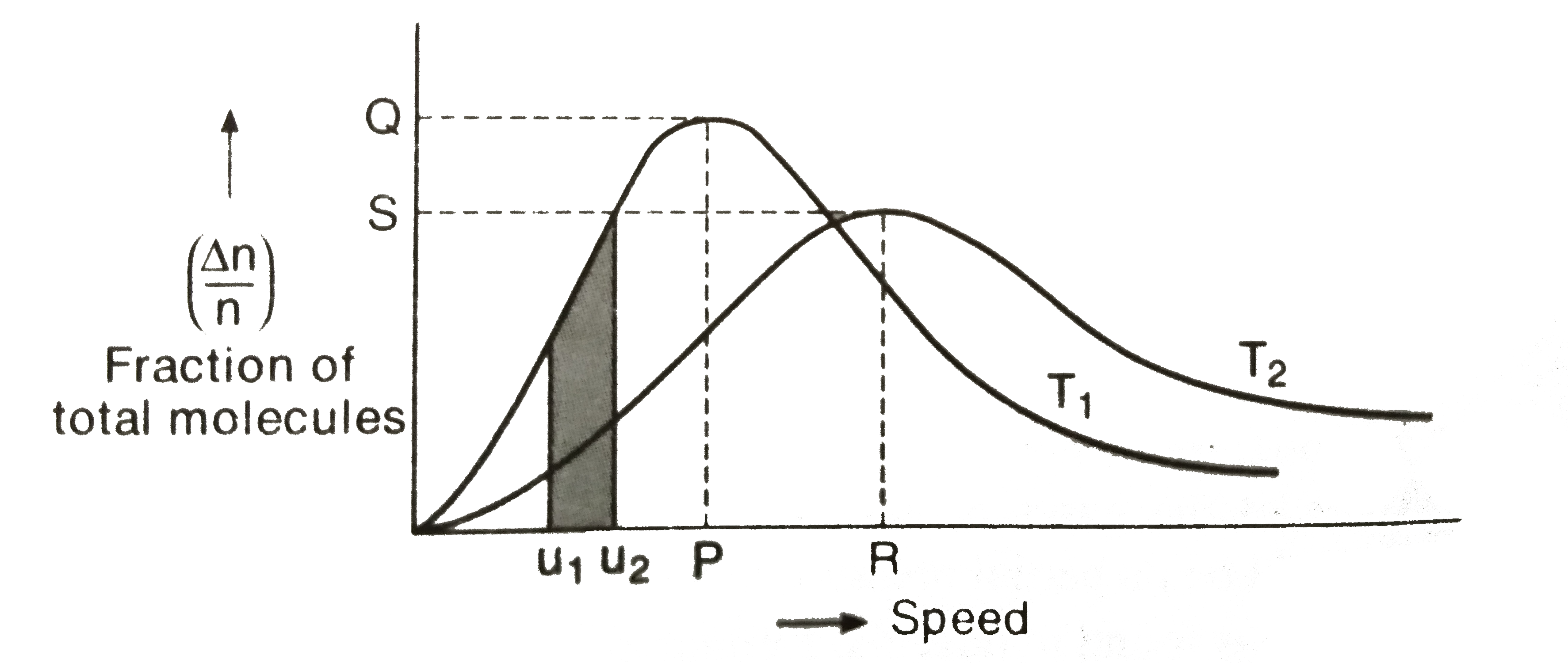
In the above graph the point 'P' refers to :
View SolutionThe relative lowering of vapour pressure of an aqueous solution containing a non-volatile solute, is 0.0125. The molality of the solution is
View SolutionThe closed packed structure have both octahedral and tetrahedral voids. In ccp structure, there is one octahedral void at the centre of body and twelve octahedral voids on the edge. Each one of which is common to four other unit cells. In ccp structure, there are eight tetrahedral voids. These voids lie on body diagonals. Two tetrahedral voids are located at each body diagonal.
The distance between an octahedral and tetrahedral void in fcc lattice would be :
View SolutionA gas can be liquefied by pressure alone when its temperature
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO2. If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called the critical pressure (Pc). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows:
Vc=3b,Pc=a27b2,Tc=8a27Rb
The relationship between Pc,Vc and Tc is :
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO_(2) . If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (T_(c)) . The pressure required to liquefy a gas at this temperature is called the critical pressure (P_(c)) . The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows: V_(c) = 3b, P_(c) = (a)/(27b^(2)), T_(c) = (8a)/(27 Rb) Which of the following parameters is three times the van der Waals' constant 'b' ?
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO2. If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called the critical pressure (Pc). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows:
Vc=3b,Pc=a27b2,Tc=8a27Rb
The critical temperature of :
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO2. If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called the critical pressure (Pc). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows:
Vc=3b,Pc=a27b2,Tc=8a27Rb
The pressure required to liquefy a gas at the critical temperature is called :
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO_(2) . If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (T_(c)) . The pressure required to liquefy a gas at this temperature is called the critical pressure (P_(c)) . The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows: V_(c) = 3b, P_(c) = (a)/(27b^(2)), T_(c) = (8a)/(27 Rb) {:("Gases",A,B,C,D,),(P_(c) (atm),2.2,14,35,45,),(T_(c) (K),5.1,33,127,140,):} Which of the above gases cannot be liquefied at 100 K and 50 atm ?
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO_(2) . If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (T_(c)) . The pressure required to liquefy a gas at this temperature is called the critical pressure (P_(c)) . The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows: V_(c) = 3b, P_(c) = (a)/(27b^(2)), T_(c) = (8a)/(27 Rb) At critical point, the meniscus between liquid and vapour disappears due to:
View SolutionThe essential conditions for liquefaction of gases were discovered by Andrews in 1869 as a result of his study of pressure-volume-temperature relationship for CO2. If was found that above a certain temperature, it was impossible to liquefy a gas whatever the pressure was applied. The temperature below which the gas can be liquefied by the application of pressure alone is called critical temperature (Tc). The pressure required to liquefy a gas at this temperature is called the critical pressure (Pc). The volume occupied by one mole of the substance at the critical temperature and pressure is called critcal volume. Critical constants are related with van der Waals' constant as follows:
Vc=3b,Pc=a27b2,Tc=8a27Rb
The values of criticla volumes of four gases A, B, C and D are 0.025 L, 0.312 L, 0.245 L and 0.432 L respectively. The gas with larger diameter will be :
View SolutionThe temperature above which no amount of pressure can cause a gas to liquefy is _____.
View SolutionThe temperature above which the gas cannot be liquefied by any amount of pressure is called…………………...
View Solution
