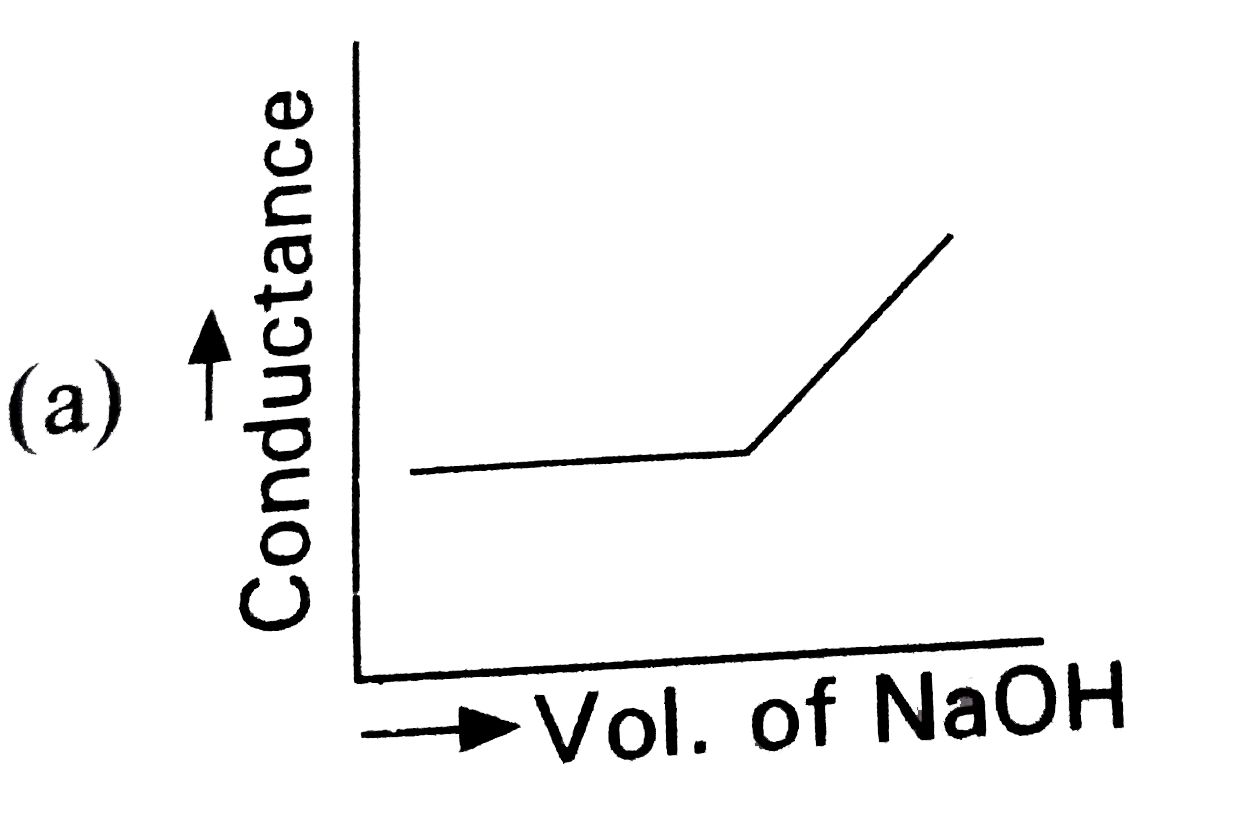
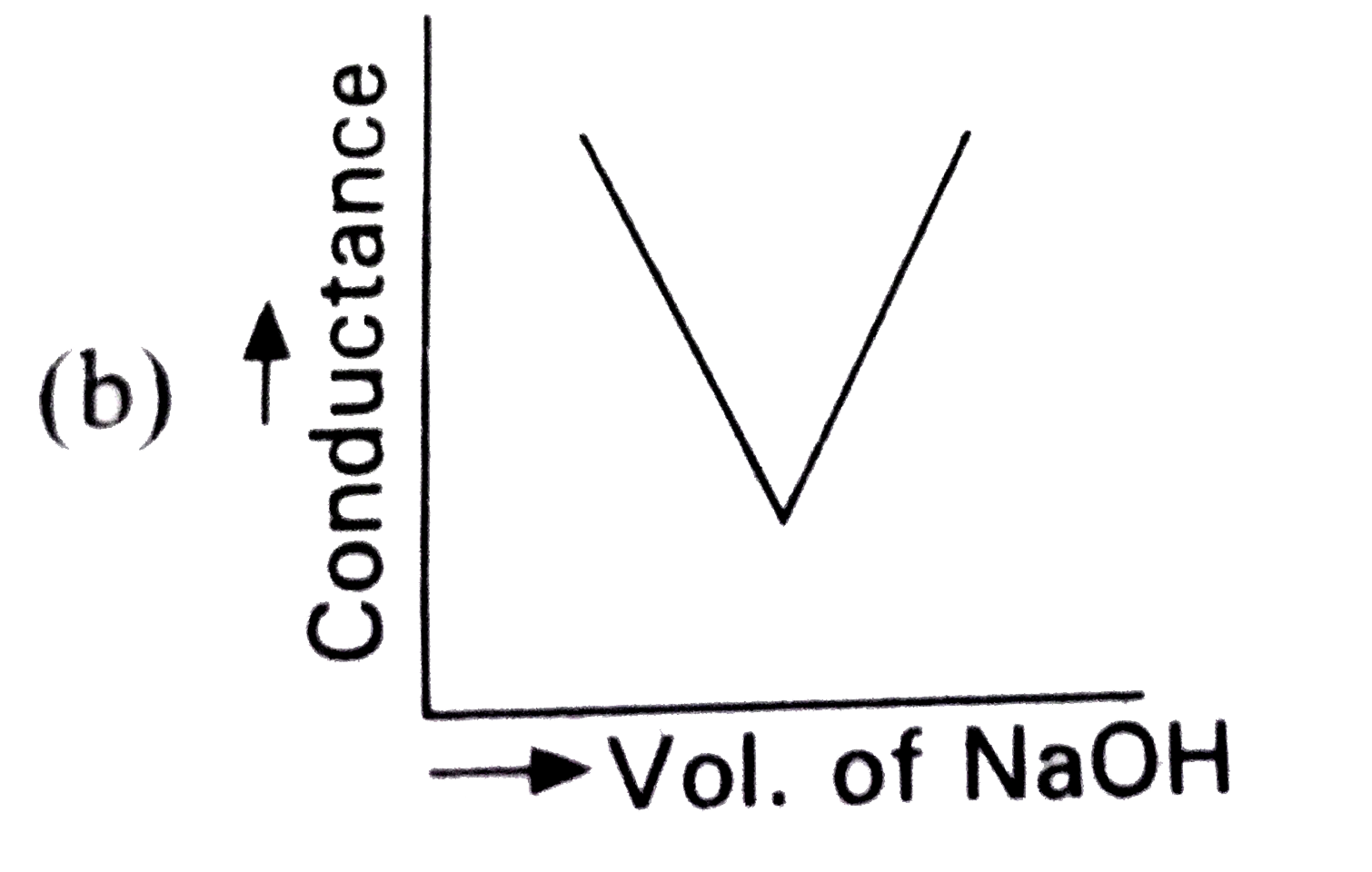
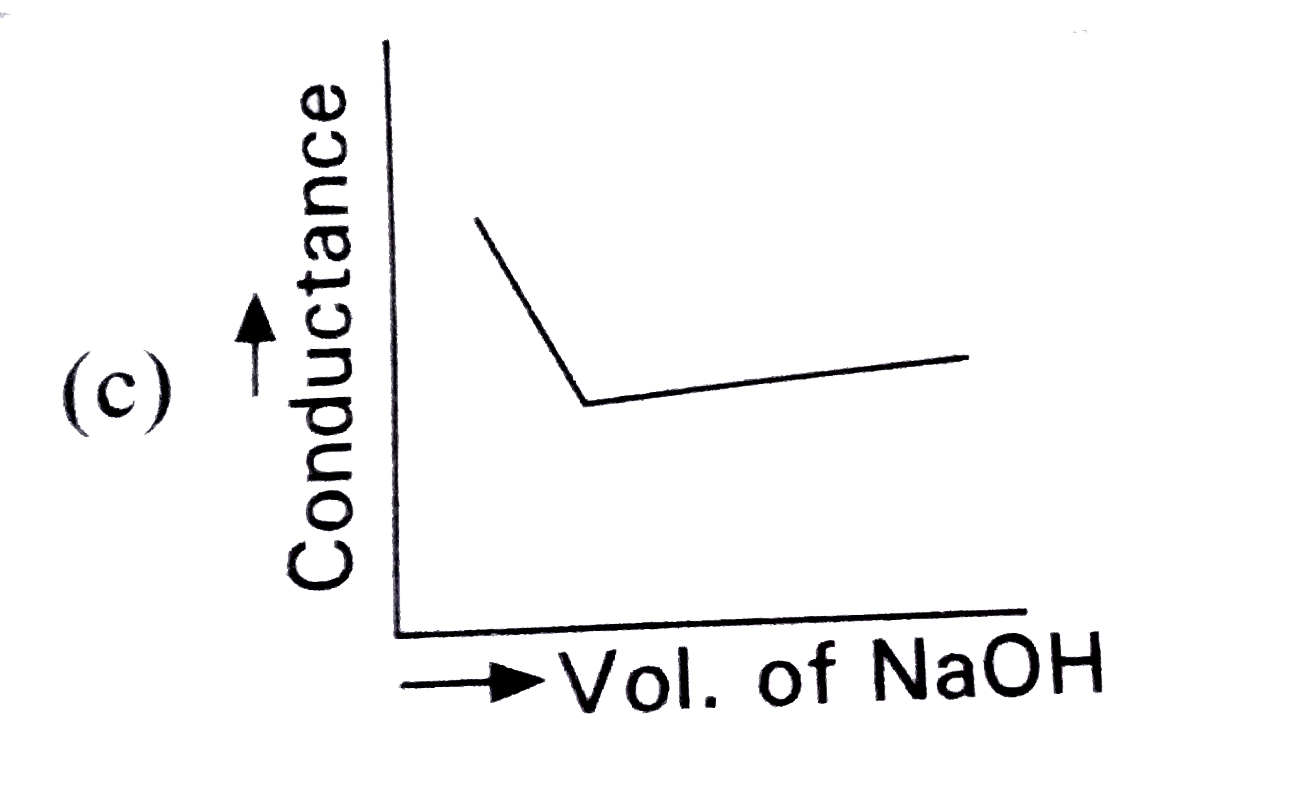
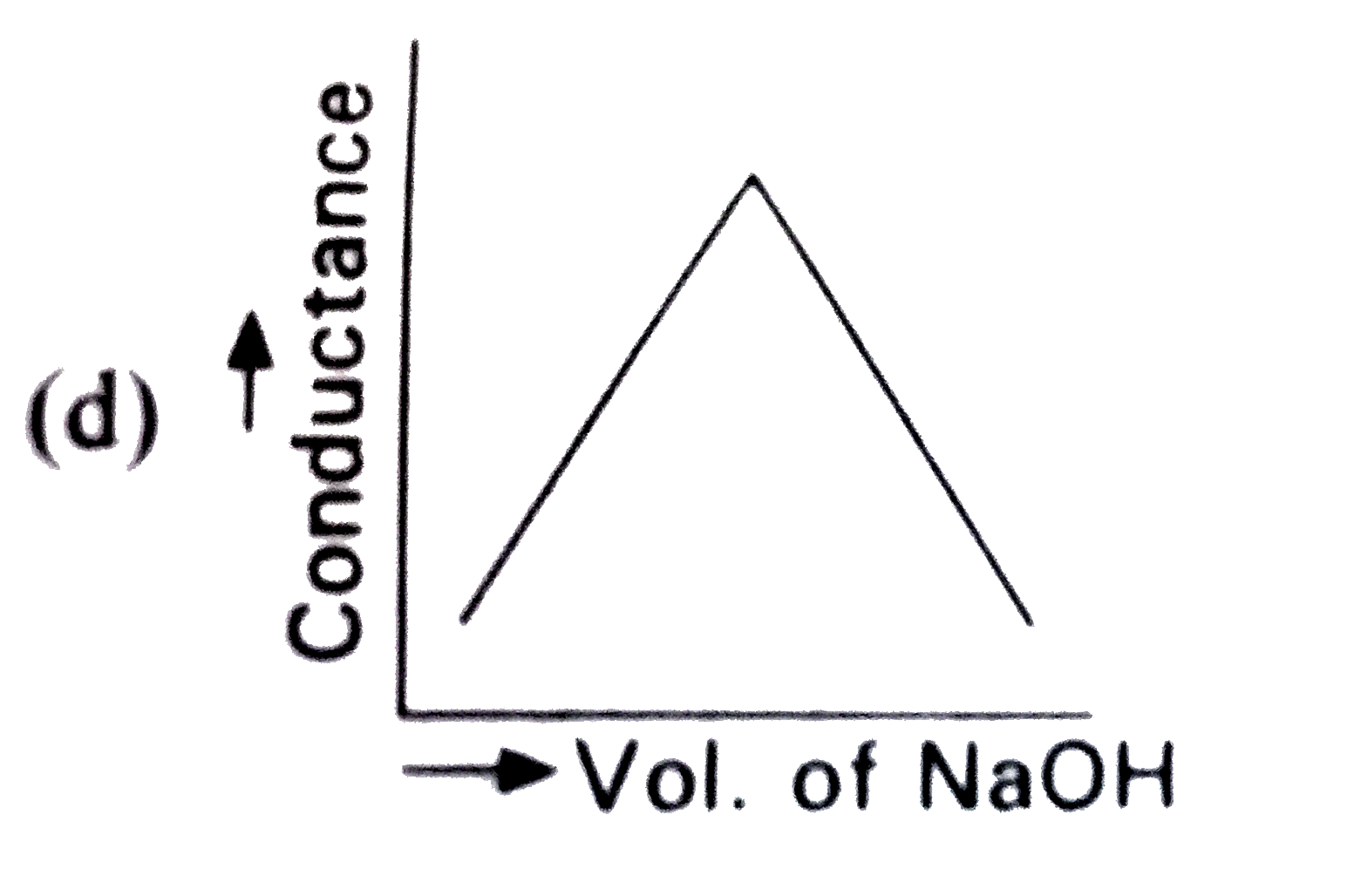
CH3COOH is neutralized by NaOH. Conductometric titration curve will be of the type:
A
B
C
D
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
CH3COOH is titrated with NaOH solution. Which is true statement?
AConductance decrease upto equivalence point, after which it increasesBConductance increases upto equivalence point, after which it decreasesCConductance first increases (but not rapidly) upto equivalence point and then increases rapidly after equivalence pointDNone of the aboveHNO3(aq) is titrated with NaOH(aq) condutomatrically, graphical representation of the titration is :
A B
B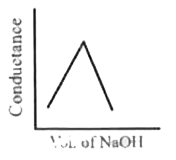 C
C D
D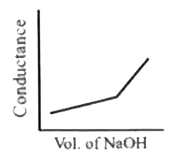
HNO3(aq) is titrated with NaOH(aq) condutomatrically, graphical representation of the titration is :
A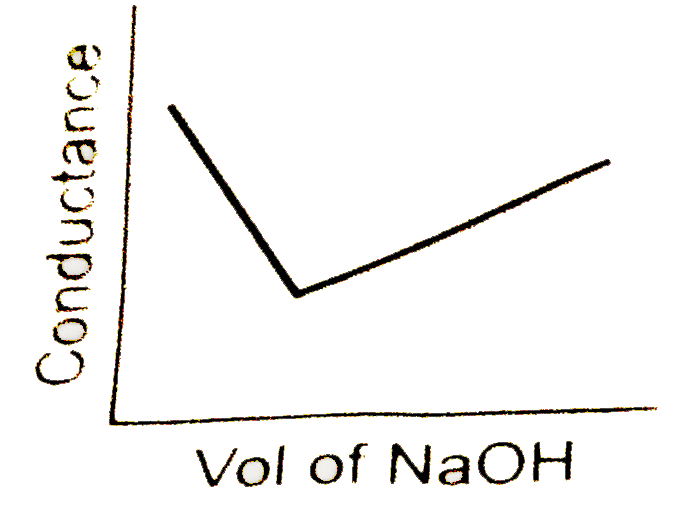 B
B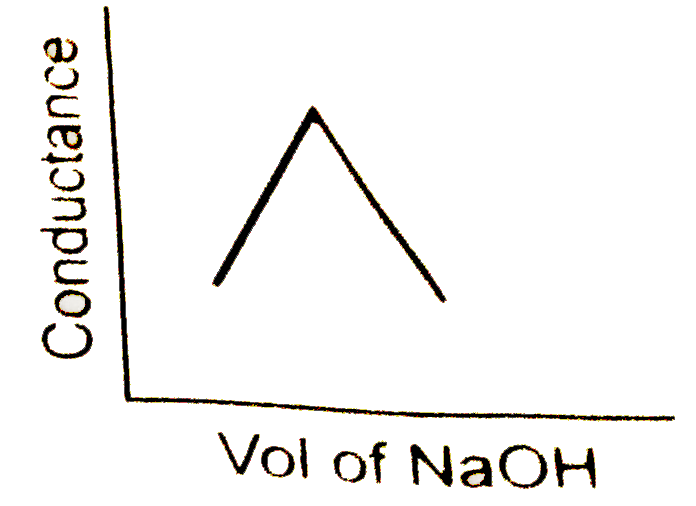 C
C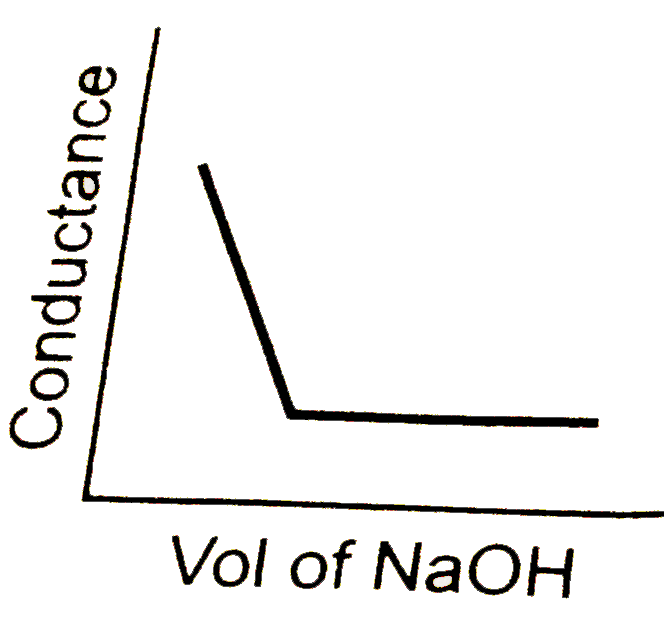 D
D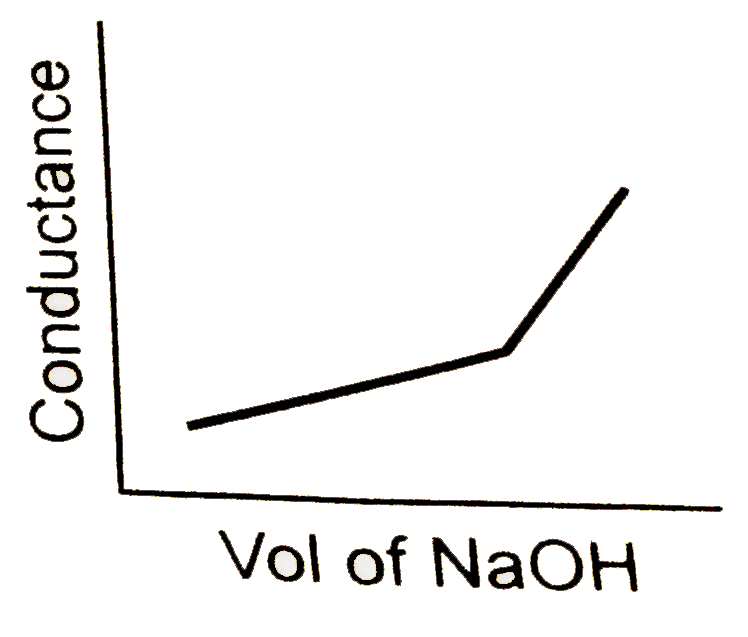
CH3COOH is titrated with NaOH solution. Which of the following statements is true ?
View SolutionHI solution is titrated with NaOH conductometrically, graphical representation of the
View SolutionWhen HCl (aq) is titrated with NaOH(aq) conductometrically then the graphical representation of the titration will be
View SolutionWhen CH3COOCH3+HCl is titrated with NaOH then at neutral point the colour of phenopthalein becomes colourless form pink due to:
View SolutionConductometric titration curve of a equimolar mixture of HCI and HCN with NaOH(aq) is
View Solution

