Match the following :
Solute (equimolar)π(OP)ratio(i)Urea, glucose, fructose(A)1:0.8:1(ii)NaCl,mgCl2,K2SO4(B)1:2:3(iii)Al2(SO4)3,Na3PO4,K4Fe(CN6)(C)1:1:1(iv)Glucose, NaCl, CaCl2(D)2:3:3
(i) (ii) (iii) (iv)A B C D
(i) (ii) (iii) (iv)B D C A
(i) (ii) (iii) (iv)D B A C
(i) (ii) (iii) (iv)C D A B
The correct Answer is:D
Step by step video, text & image solution for Match the following : {:(,"Solute (equimolar)",,pi"(OP)ratio"),("(i)","Urea, glucose, fructose","(A)",1:0.8:1),("(ii)","NaCl,mgCl"_(2)",K"_(2)"SO"_(4),"(B)",1:2:3),("(iii)",Al_(2)(SO_(4))_(3)","Na_(3)PO_(4)","K_(4)Fe(CN_(6)),"(C)",1:1:1),("(iv)","Glucose, NaCl, CaCl"_(2),"(D)",2:3:3):} by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Column-IColumn-IIelectrolyteI(van't off factor)(A)Urea, Glucose, Fructose(p)1:0.8:1(B)NaCl,MgCl2,K2SO4(q)1:2:3(C)Al2(SO4)3Na3PO4,K4[Fe(CN)6](r)1:1:1(D)Glucose,NaCl,CaCl2(s)2:3:3
View Solution[G] Match the salts in Column-I with the ratio of van't Hoff factor in Column-II :
(,Column-I,,Column-II),((a),Glucose, fructose, sucrose,(p),1:2:3),((b),KCl,MgCl2,FeCl3,(q),1:3:4),((c),K4[Fe(CN)6],Na3PO4,Al2(SO4)3,(r),2:3:4),((d),Urea, KCl, CaCl2,(s),1:1:1)
[Note : Assume that the electrolytes are completely ionised.
View Solution
Knowledge Check
Na3PO4+Fe2(SO4)3→FePO4⏐↓+Na2SO4
AFor coloured ppt./Black pptBFor coloured solution.Cfor clear/colourless solutionDFor white ppt.Among 0.1M solution of NH2CONH2,Na2PO4 and Al2(SO4)3:
AThe V.P. and freezing point are the highest for urea.BThe elevation in boiling point is the highest for Al2(SO4)3CThe V.P. and freezing point is the lowest for ureaDThe depression in freezing point in the highest for the Al2(SO4)3Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code.
Column I (Compound)Column I (Oxidation state of Co)A.[Co(NCS)(NH3)5](SO3)1.+4B.[Co(NH3)4Cl2]SO42.0C.Na4[Co(S2O3)3]3.+1D.[Co2(CO)8]4.+25.+3AA (1)B (2)C (4)D (5)BA (4)B (3)C (2)D (1)CA (5)B (1)C (4)D (2)DA (4)B (1)C (2)D (3)
[A] Match the solutes in Column-I with th van't Hoff factors in Column-II :
Column-IColumn-II(a)K4[Fe(CN)6](p)1+α(b)Al2(SO4)3(q)Greater than 1(c)NH2−O||C−NH2(r)(1+4α)(d)CaCl2(s)1
α= Degree of ionization.
View SolutionMatch the following Columns.

ABCD(a)1324(b)3241(c)4321(d)4231
View SolutionCalculate the O.N of
(i)CrinK2CrO4
(ii)SinH2SO4
(iii)SinNaHSO4
NinHNO3
(iv)PinH3PO4
View SolutionMatch the following Columns
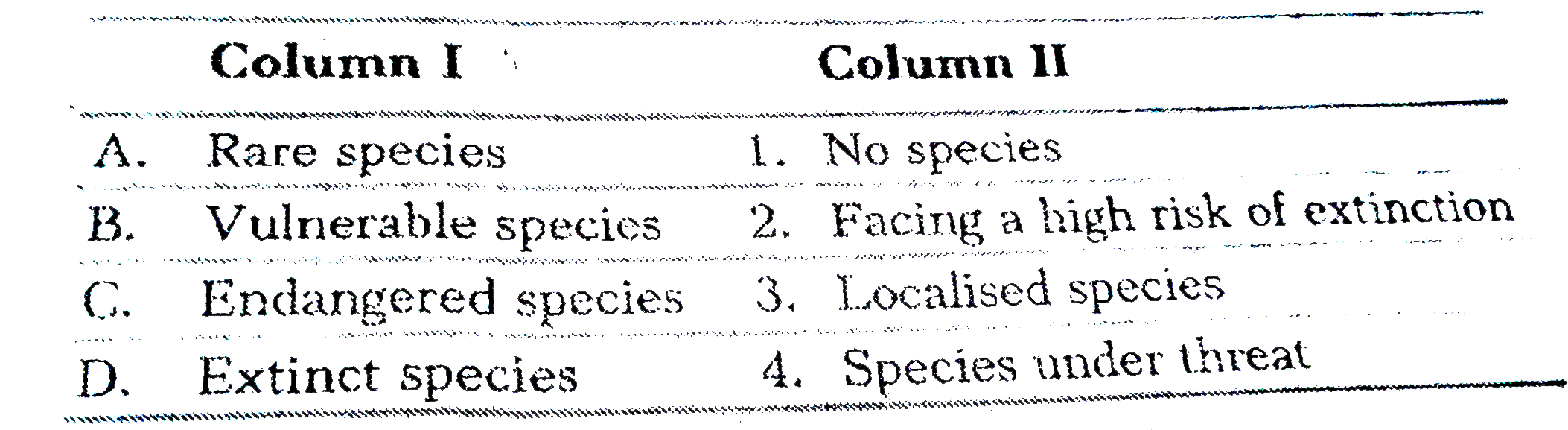
View SolutionMatch the following Columns

ABCD(a)4321(b)1243(c)3412(d)2143
View Solution
