Explain the following with proper reasoning :
(a) Although benzene is highly unsatureted, it does not undergo addition reactions.
(b) Benzene though unsaturated, undergoes substitutions reactions easily reather than addition.
( c) All m-directors are deactivating.
(d) The halogens, as exceptions, are o-p-directors but are deactivating.
( e) Most o- p-directing substituents are activating.
(a) π=electrons of benzene ring are dilocali-zed throughout the molecule. This makes the molecule very stable. The addition reactions would result in the breaking of this delocalization, i.e., the stability of the molecule which is resisted.
(b) Resonace is present in benzene molecule. This makes the benzene molecule stable. As the substitution reactions do not disturb the resonance stabilization or aromatic character, thus, there reactions are resisted.
( c) The meta-directing substituents withdraw electrons form the benzene ring and thus, deactivate the benzene ring for further substitution. (d) In the case of halogens, two opposing effects operate simultaneously. A halogen substitument releases electrons due to resonance but withdraw wlwtrons also due to high electronegativityt, i.e., -I (inductive) effect. The inductive effect dominates more than the resonance effect. The inductive effect dominates more than the resonance effect and the net result is that the benzene ring is redered electron deficient and thus deactivated for further substitution.
( e) the o-, p-directing substituents release eletrons into the benzene ring and thus, activate the banzene ring for further substitution.
Topper's Solved these Questions
Similar Questions
Give reason for the following in one or two sentences:
'Although benzene in highly unsaturated, normally it does not undergo addition reaction'.
View SolutionGive reason for the following in one or two sentences:
'Although benzene in highly unsaturated, normally it does not undergo addition reaction'.
View Solution
Knowledge Check
Although benzene is highly unsaturated it does not undergo addition reactions. The explanation of this can be suggested as
Aπ-electrons of benzene ring are delocalisedBsince π-electrons are present inside the ring, addition cannot take placeCcyclic structures do not show addition reactionsDbenzene is not a reactive compound.The typical reaction of benzene and other aromatic compounds are electrophilic substitution. Presence of electron donating group activates the ring towards electrophilic substitution, while presence of electron withdrawing group deactivates the ring towards electrophilic substituion but at the same time activates the ring towards nucleophilic subsituion. Some groups are predominantly meta-directing and all of these are deactivating. Except halogen, most of the o−andp−directing groups are activating groups.
_E01_246_Q01.png) C2H5Cl.AlCl3−−−−−−−−→Δ(A) major.
C2H5Cl.AlCl3−−−−−−−−→Δ(A) major.
A is trisubstituted benzene. The structure of A is :A_E01_246_O01.png) B
B_E01_246_O02.png) C
C_E01_246_O03.png) D
D_E01_246_O04.png)
The typical reaction of benzene and other aromatic compounds are electrophilic substitution. Presence of electron donating group activates the ring towards electrophilic substitution, while presence of electron withdrawing group deactivates the ring towards electrophilic substituion but at the same time activates the ring towards nucleophilic subsituion. Some groups are predominantly meta-directing and all of these are deactivating. Except halogen, most of the o−andp−directing groups are activating groups.
Which of the following compound is not formed.
_E01_248_Q01.png) (i)NBS,CCl4,hv−−−−−−−−−−−→(II)Br2/FeBr3(X)
(i)NBS,CCl4,hv−−−−−−−−−−−→(II)Br2/FeBr3(X)
X represents mixture of organic compounds. The mixture does not containA_E01_248_O01.png) B
B_E01_248_O02.png) C
C_E01_248_O03.png) Dall of them
Dall of them
Explain the following:
(a) Ethylamine is soluble in water, whereas aniline is not.
(b) Although amino group is o- and p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(c) Aniline does not undergo Friedel Crafts reaction.
View SolutionGive reasons for the following:
(i) tert-buty lbenzene does not give benzonic acid on treatment with acidic KMnO4.
(ii)Normally, benzene gives electrophilic substitution reaction rather than electrophilic addition reaction although it has double bond.
View Solution(a) Explain why haloarenes undergoes electrophilic substitution reactions at ortho and para positions only
(b) The p-isomer of dichloro benzene has higher melting point than its ortho and meta-isomers. Explain.
View Solution(a) Explain why haloarenes undergoes electrophilic substitution reactions at ortho and para positions only
(b) The p-isomer of dichloro benzene has higher melting point than its ortho and meta-isomers. Explain.
View SolutionExplain the following with proper reasonin
(i) Why do halogen acids which are easily added to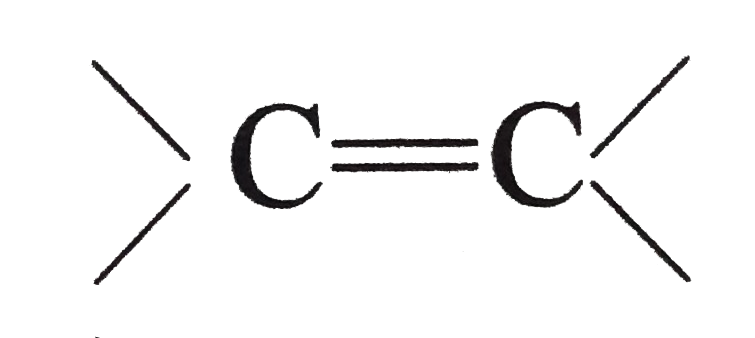
(ii) Pure HCN fails to react with aldehydes.
(iii) Why only RCHO, RCOCH3 and cyclic ketones react with NaHSO3?
(iv) Chloral hydrate is a stable compound even it is a gem-diol.
(v) Formation of oximes and other ammonia derivatives from carbonyl compounds require slightly acidic media for maxiumum rate
(vi) In the following reaction, three isomers are present in the equilibrium which is most stable
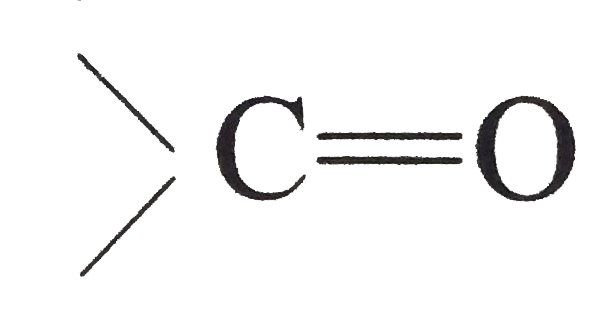
(vii) teh strurcture of two isomeric oximes formed by the reaction of acetophenon with hydroxylamine hydrochloride.
(viii) Why o-hydroxy benzaldehyde is a liquid at room temperature while p-hydroxy benzaldehyde is high metling solid?
View Solution

