The diamagenetic species is :
A
NO
B
NO2
C
O2
D
CO2
Video Solution
Text Solution
The correct Answer is:D
Species having unpaired electron are paramagnetic & without unpaired are diamagnetic
: N = O1 unpaired eletron O22unpairedMOT−
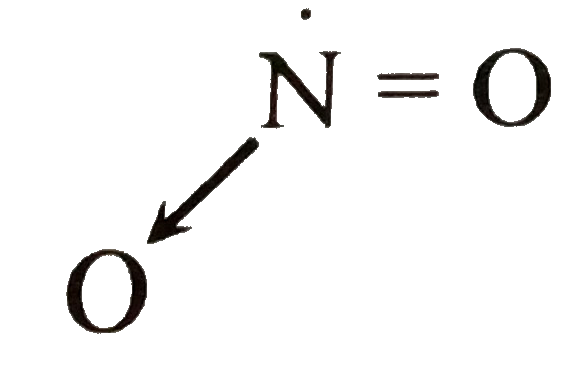 1 unpaired electron" " CO_(2) no unpaired electron
1 unpaired electron" " CO_(2) no unpaired electron
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
The diamagnetic species is
A[Ni(CN)4]2−B[NiCl4]2−C[CoCl4]2−D[CoF6]2−The paramagnetic species is
AKO2BSiO2CTiO2DBaO2Species is a
Agroup immediately below a phylmBclosely related interbreeding populationCtaxonamic division of similar generaDclosely related non- breeding population
The origin of new species from an existing species is called_____.
View SolutionAssertion :- All diamagnetic species are colorless
Reason :- All paramagnetic species are colorful.
View SolutionParamagnetic species are
View SolutionParamagnetic species are
View SolutionNeontological species is
View Solution
