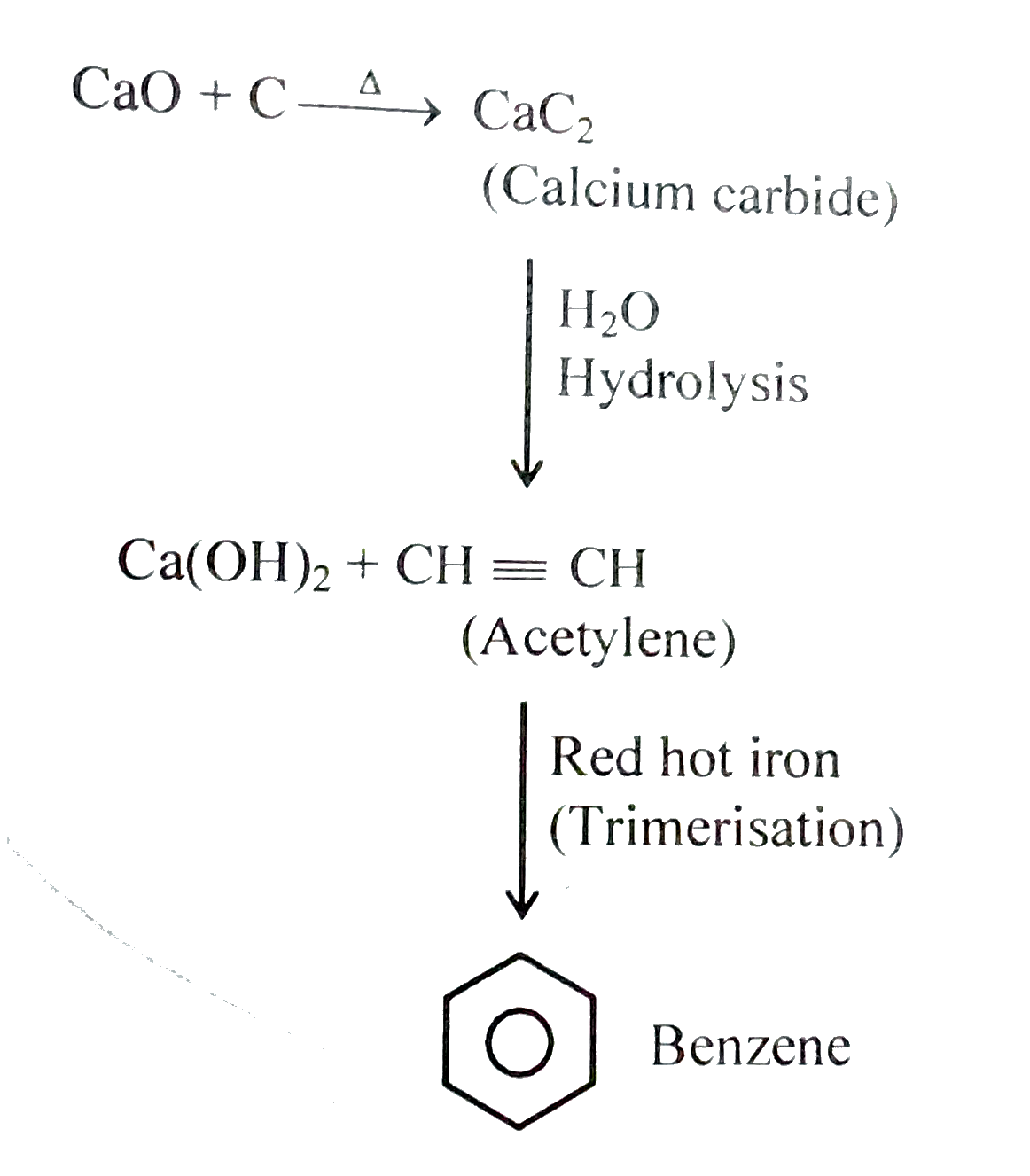
A compound X formed after heating coke with lime react with water to give Y which on passing over redhot iron at 873 produces Z. The compound Z is
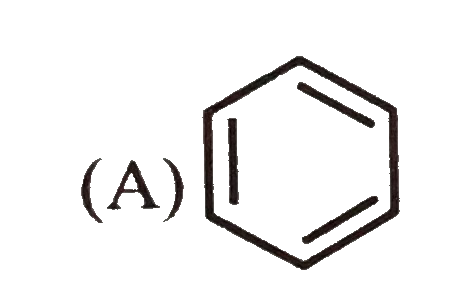
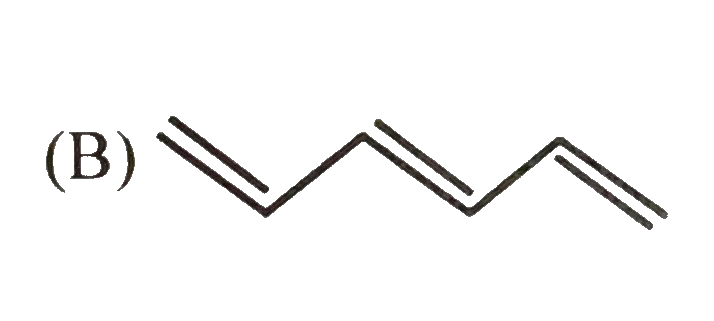
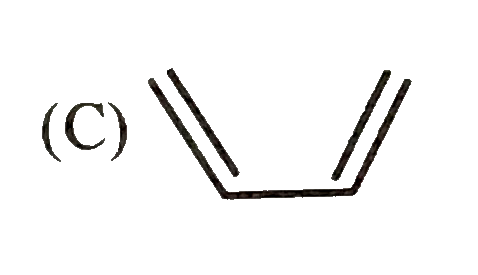
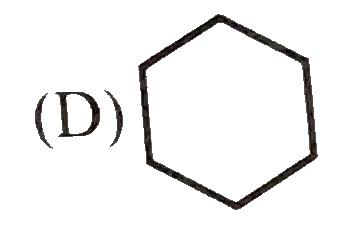
The correct Answer is:A
Similar Questions
(A) light blue coloured compound on heating will convert into black (B) which reacts with glucose gives red compound (C) and (A) reacts with ammonium hdyroxide in excess in presence of ammonium sulphate give blue compound (D). What is (A), (B), (C) and (D)?
View SolutionAn organic compound [A] is C5H8O3.On heating with soda lime, it gives [B]. The compound [B] reacts with HCN to give another compound [C] which reacts with thionyl chloride to produce [D]. When the compound [A] is heated with potassium dichromate the product is acetic acid and malonic acid.
The structure of compound [A] is :
View Solution
Knowledge Check
Which compound is formed when iron reacts with carbon?
AFeC2BFe3CCFeC3DFe2CWhich compound is formed when iron reacts with carbon?
AFeC2BFe3CCFeC3DFe2CAn organic compound [A] is C5H8O3.On heating with soda lime, it gives [B]. The compound [B] reacts with HCN to give another compound [C] which reacts with thionyl chloride to produce [D]. When the compound [A] is heated with potassium dichromate the product is acetic acid and malonic acid.
The compound [A] is aAα-hydroxy acidBβ-hydroxy esterCβ-keto acidDβ-keto ester.
Compound 'A' is reacted with HX produces 'B' which is heated with silver nitrate gives 2-nitropropane. The compound 'A' is
View SolutionWhen a compound X reacts with ozone in aq medium, a compound Y is produced. Ozone also reacts with moist Y and produces compound Z. Z acts as an oxidising agent, then X, Y and Z will be:
View SolutionA compound of calcium 'X' is used in the purification of water. It reacts with water to form another compound 'Y' with the evolution of gas 'Z'. When carbon dioxide gas is passed through 'Y', it turns milky. Gas 'Z' helps in the purification of water.
Which of the following statements regarding the given piece of information is correct?
View SolutionA primary alcohol, C3H8O(W) on heating with sulphuric acid undergoes dehydration to give an alkene , (X), which reacts with HCl to give (Y). (Y) on treatment with aqueous KOH gives compound (Z),C3H8O. Compounds (W) and (Z) are :
View SolutionA compound 'X' when reacted with PCI5 and then with NH3 gives 'Y'. When 'Y' treated with Br2 and KOH produced 'Z'. Z on treatement with NaNO2+HCI at 0∘C and then boiling produced ortho-cresol. Compound 'X' is:
View Solution