Which of the following reaction is possible at the angle?
A
2Cr2++7H2O→Cr2O2−7+14H+
B
F2→2F−
C
12O2+3H+→H2O
D
None of these
Video Solution
Text Solution
The correct Answer is:A
Oxidation (loss of electrons)takes place at anode i.e., (A).
|
Topper's Solved these Questions
- ELECTROCHEMISTRYBOOK - DINESH PUBLICATIONCHAPTER - ELECTROCHEMISTRYEXERCISE - SELECTED STRAIGHT OBJECTIVE
67 Videos
- ELECTROCHEMISTRYBOOK - DINESH PUBLICATIONCHAPTER - ELECTROCHEMISTRYEXERCISE - MATCH-MATRIX
7 Videos
- ELECTROCHEMISTRYBOOK - DINESH PUBLICATIONCHAPTER - ELECTROCHEMISTRYEXERCISE - ADDITIONAL NUMERICAL PROBLEMS FOR PRACTICE
12 Videos
- D-AND -F BLOCK ELEMENTSBOOK - DINESH PUBLICATIONCHAPTER - D-AND -F BLOCK ELEMENTSEXERCISE - BRAIN STORMING MULTIPLE CHOICE QUESTIONS (MCQS)
13 Videos
- ETHERSBOOK - DINESH PUBLICATIONCHAPTER - ETHERSEXERCISE - (MCQs)
8 Videos
Similar Questions
Knowledge Check
Which of the following reactions is possible?
A2KBr+Cl2→2KCl+Br2B2Kl+Br2→2KBr+l2C2Kbr+l2→2Kl+Br2D2H2O+2F2→4HF+O2Which of the following reactions is possible ?
AC6H5OH+HBr→C6H5Br+H2OB(CH3)3CCl+NaOCH3→(CH3)3COCH3+NaClC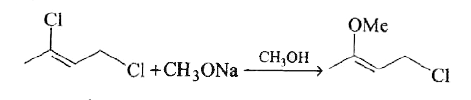 D
D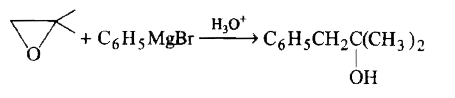
Which of the following reactions is possible ?
A2KBr+Cl2→2KCl+Br2B2Kl+Br2→2KBr+l2C2Kbr+l2→2Kl+Br2D2H2O+2F2→4HF+O2
Which of the following reactions is possible ?
View SolutionWhich of the following reaction is possible at anode?
View SolutionWhich of the following reaction is not possible
View SolutionWhich of the following reaction is not possible
View SolutionWhich of the following reaction is/are possible
View Solution

