Van't Hoff factoe (i) is the ratio of
A
Observed molecular mass to calculated molecular mass
B
Obserbed colligative property to calculated value
C
Calculated colligative property to obserbed value
D
Number of moles dissolved to the total number of moles taken
Video Solution
Text Solution
The correct Answer is:B
I=Obsd.Colligative propertyNormal
∴i>1
,' dissociation increases particles in solution.
|
Topper's Solved these Questions
- SOLUTIONS BOOK - DINESH PUBLICATIONCHAPTER - SOLUTIONS EXERCISE - REVISION QUESTION
186 Videos
- SOLUTIONS BOOK - DINESH PUBLICATIONCHAPTER - SOLUTIONS EXERCISE - OBJECTIVE TYPE MCQs
47 Videos
- SOLID STATEBOOK - DINESH PUBLICATIONCHAPTER - SOLID STATEEXERCISE - Brain storming
10 Videos
- SOME BASIC CONCEPTS OF CHEMISTRYBOOK - DINESH PUBLICATIONCHAPTER - SOME BASIC CONCEPTS OF CHEMISTRYEXERCISE - ULTIMATE PREPARATORY PACKAGE
20 Videos
Similar Questions
Knowledge Check
van't Hoff factor (i)
AIs less than one in case of dissociationBis more than one in case of associationCi=normal molecular mass /oberved molecular massDi=observed molecular mass/normal molecular massWhich of the following salt will have the same value of Van't Hoff's factor (i) as that of K4[Fe(CN)6]
AAl2(SO4)3BNaClCAl(NO3)2DNa2SO4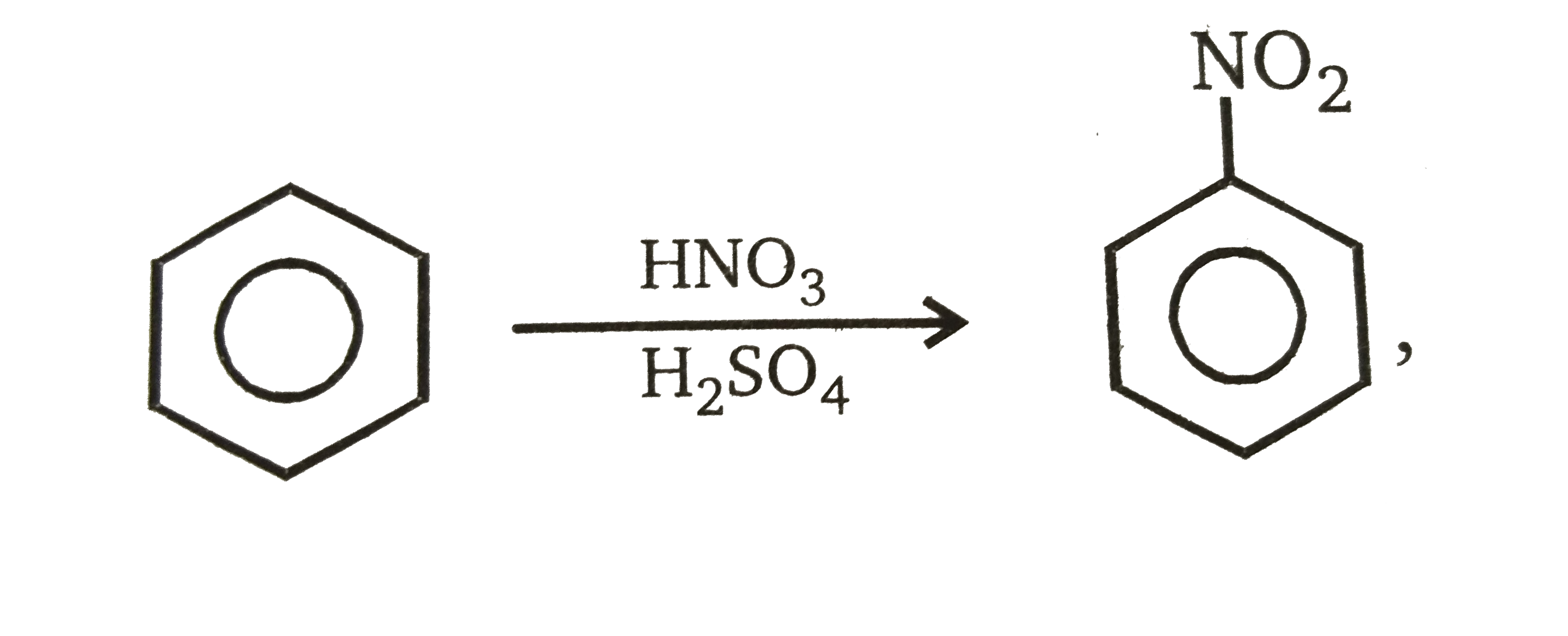 , van't -Hoff factor (i) for this reaction is:A2B3C4D5
, van't -Hoff factor (i) for this reaction is:A2B3C4D5
v1_newFlow
View SolutionThe degree of dissociation (α) of a weak electrolyteAxBy is related to Van't Hoff factor
(i) by the expression ______________.
View SolutionBenzoic acid undergoes dimerisation in benzene solution. The van't Hoff factor (i) is related to the degree of association (α) of the acid as
View SolutionVan't Hoff factor is _____.
View SolutionWhich of the following compounds corresponds to Van't Hoff factor ( i) to be equal to 2 for dilute solution ?
View Solution

