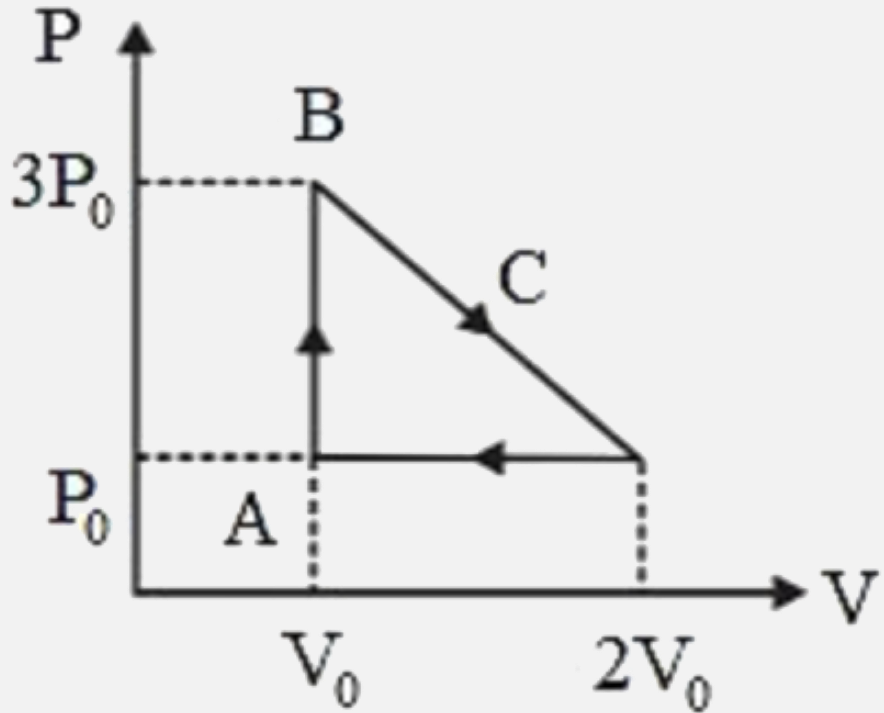
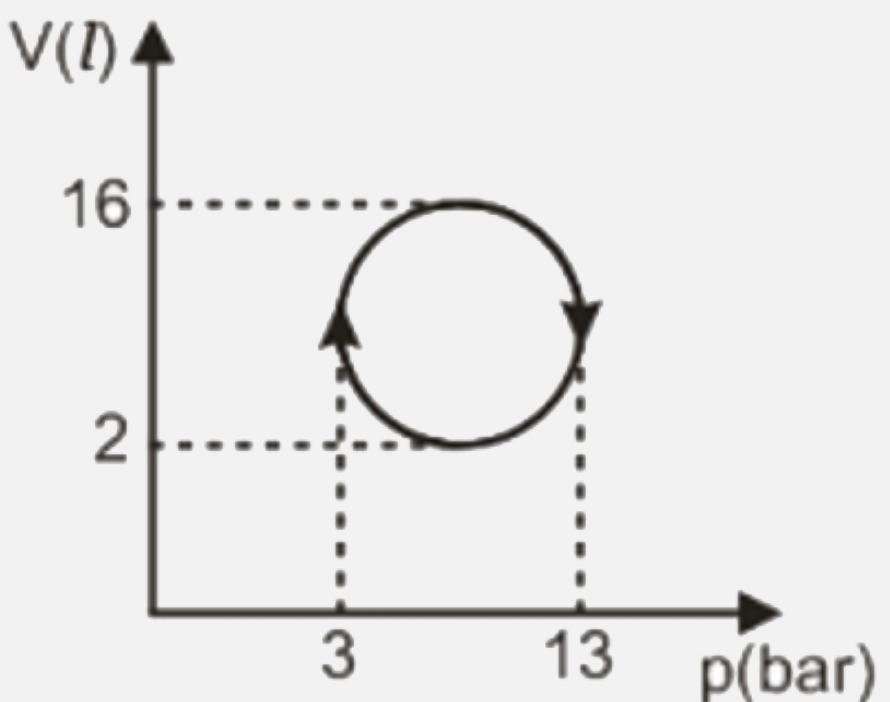
The work done by the system in a cyclic process involving one mole of an ideal monoatomic gas is −50kJ/cyc≤. The heat absorbed by the system per cycle is
Zero
50kJ
−50kJ
250kJ
The correct Answer is:B
To find the heat absorbed by the system per cycle in a cyclic process involving one mole of an ideal monoatomic gas, we can use the first law of thermodynamics. Here’s the step-by-step solution:
Step 1: Understand the First Law of Thermodynamics
The first law of thermodynamics states that:
ΔU=Q+W
where:
- ΔU is the change in internal energy,
- Q is the heat absorbed by the system,
- W is the work done by the system.
Step 2: Identify the Characteristics of a Cyclic Process
In a cyclic process, the system returns to its initial state, which means that the change in internal energy (ΔU) is zero:
ΔU=0
Step 3: Apply the First Law to the Cyclic Process
Since ΔU=0, we can rewrite the first law as:
0=Q+W
This implies:
Q=−W
Step 4: Substitute the Given Work Done
We are given that the work done by the system is:
W=−50kJ
Now, substituting this value into the equation for Q:
Q=−(−50kJ)=50kJ
Step 5: Conclusion
The heat absorbed by the system per cycle is:
Q=50kJ
Final Answer
The heat absorbed by the system per cycle is 50 kJ.
---
The first law of thermodynamics states that:
ΔU=Q+W
where:
- ΔU is the change in internal energy,
- Q is the heat absorbed by the system,
- W is the work done by the system.
In a cyclic process, the system returns to its initial state, which means that the change in internal energy (ΔU) is zero:
ΔU=0
Since ΔU=0, we can rewrite the first law as:
0=Q+W
This implies:
Q=−W
We are given that the work done by the system is:
W=−50kJ
Now, substituting this value into the equation for Q:
Q=−(−50kJ)=50kJ
The heat absorbed by the system per cycle is:
Q=50kJ
The heat absorbed by the system per cycle is 50 kJ.
Similar Questions
Knowledge Check
What would be the heat absorbed by the system in the cyclic process?
A−2PVBzeroC2PVDPV550kJcycle−1 work is done by 1 mol of an ideal gas in a cyclic process. The amount of heat absorbed by the system in one cycle is
A550 kJB−550kJC1100 kJDZeroIn a cyclic process, work done by the system is
AzeroBmore than the heat given to the systemCequal to heat given to the systemDinedpendent of heat given to the system
In a cyclic process, work done by the system is
View SolutionWork done (in kJ) by the gas in the following cyclic process is

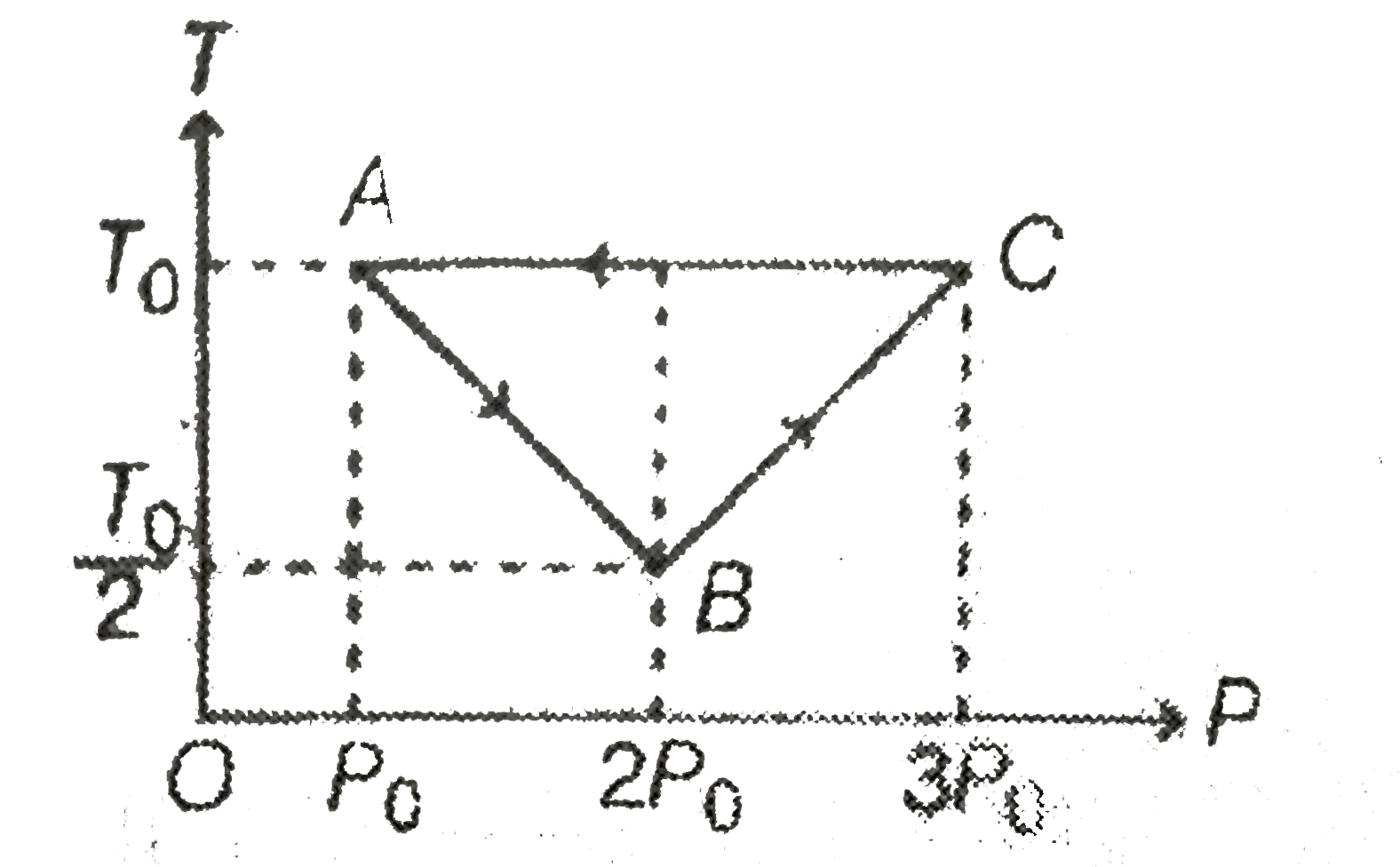
View Solutionp-T diagram of one mole of an ideal monatomic gas is shown. Processes AB and CD are adiabatic. Work done in the complete cycle is

View SolutionOne mole of an ideal monoatomic gas is taken through a cyclic process as shown. Choose the correct option(s).
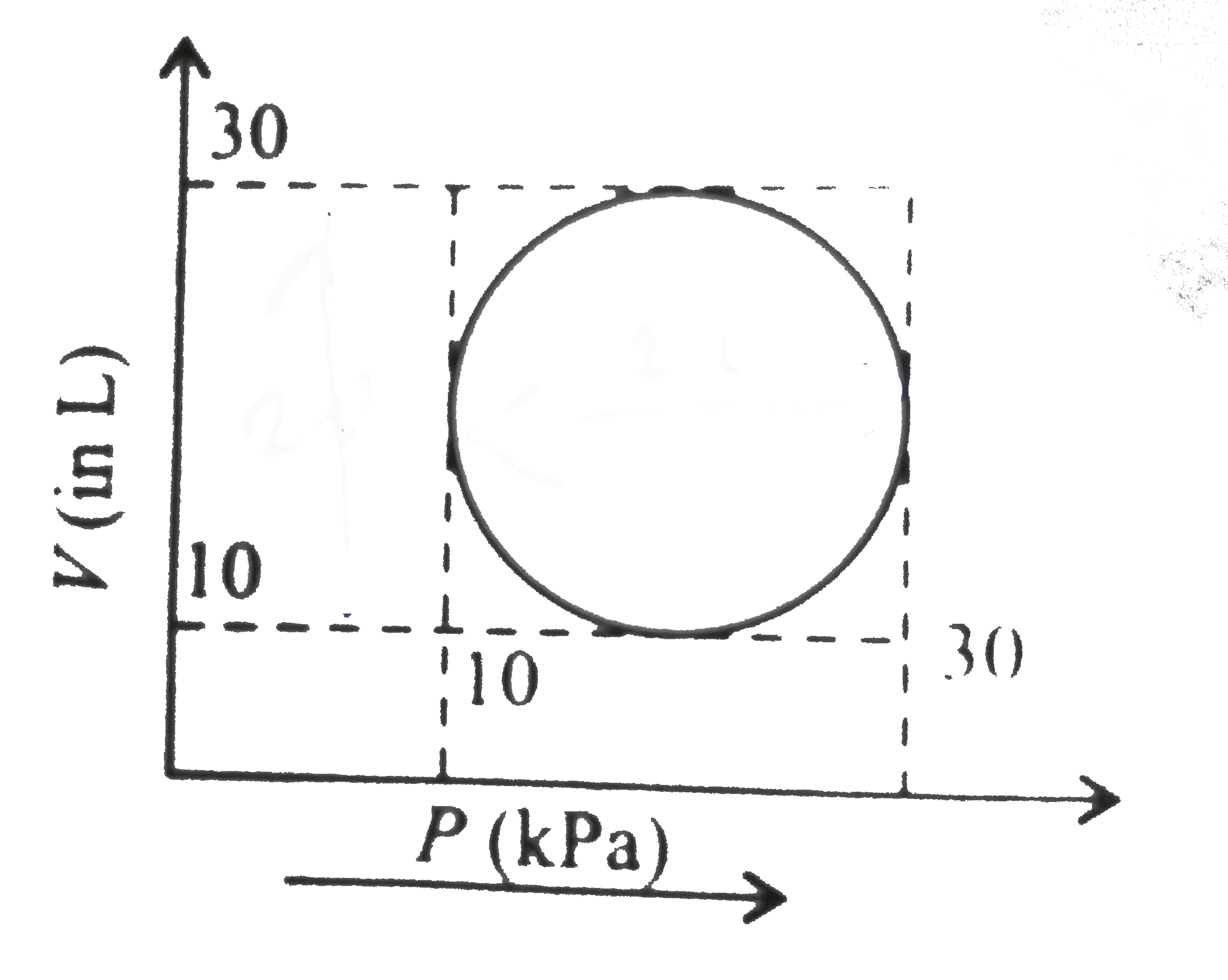
View SolutionHeat energy absorbed by a system in going through a cyclic process shown in figure is
View Solution