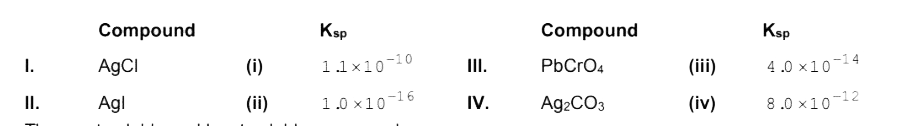The solubility of product (Ksp) of the following compounds are given at 25∘C
CompoundKspCompoundKspAgCl1.1×10−10PbCrO44.0×10−4AgI1.0×10−16Ag2XO38.0×10−12
The most soluble and least soluble compounds are respectively
AgCl and PbCrO4
AgI and Ag2CO3
AgCl and Ag2CO3
Ag2CO3 and PbCrO4
The correct Answer is:D
To determine the most soluble and least soluble compounds from the given solubility products (Ksp) at 25°C, we can follow these steps:
Step 1: List the given compounds and their Ksp values
We have the following compounds and their corresponding Ksp values:
- AgCl: Ksp=1.1×10−10
- AgI: Ksp=1.0×10−16
- PbCrO4: Ksp=4.0×10−4
- Ag2XO3: Ksp=8.0×10−12
Step 2: Understand the relationship between solubility and Ksp
The solubility product (Ksp) indicates the extent to which a compound can dissolve in water. A higher Ksp value means that the compound is more soluble, while a lower Ksp value indicates lower solubility.
Step 3: Compare the Ksp values
Now, we will compare the Ksp values of the compounds:
- AgCl: 1.1×10−10
- AgI: 1.0×10−16
- PbCrO4: 4.0×10−4
- Ag2XO3: 8.0×10−12
Step 4: Identify the most soluble compound
The compound with the highest Ksp value is PbCrO4, which has a Ksp of 4.0×10−4. Therefore, PbCrO4 is the most soluble compound.
Step 5: Identify the least soluble compound
The compound with the lowest Ksp value is AgI, which has a Ksp of 1.0×10−16. Therefore, AgI is the least soluble compound.
Conclusion
The most soluble compound is PbCrO4 and the least soluble compound is AgI.
---
We have the following compounds and their corresponding Ksp values:
- AgCl: Ksp=1.1×10−10
- AgI: Ksp=1.0×10−16
- PbCrO4: Ksp=4.0×10−4
- Ag2XO3: Ksp=8.0×10−12
The solubility product (Ksp) indicates the extent to which a compound can dissolve in water. A higher Ksp value means that the compound is more soluble, while a lower Ksp value indicates lower solubility.
Now, we will compare the Ksp values of the compounds:
- AgCl: 1.1×10−10
- AgI: 1.0×10−16
- PbCrO4: 4.0×10−4
- Ag2XO3: 8.0×10−12
The compound with the highest Ksp value is PbCrO4, which has a Ksp of 4.0×10−4. Therefore, PbCrO4 is the most soluble compound.
The compound with the lowest Ksp value is AgI, which has a Ksp of 1.0×10−16. Therefore, AgI is the least soluble compound.
The most soluble compound is PbCrO4 and the least soluble compound is AgI.
Similar Questions
Knowledge Check
The solubility product (Ksp) of the following compounds are given at 25∘C
CompoundKspAgCl1.1×10−10Agl1.0×10−16PbCrO44.0×10−14Ag2CO38.0×10−12
The most soluble and least soluble compound are respectivelyAAgClandPbCrO4BAgIandAg2CO3CAgClandAg2CO3DAg2CO3andAgIThe solubility product (Ksp) of the following compounds are given at 25∘C
CompoundKspAgCl1.1×10−10Agl1.0×10−16PbCrO44.0×10−14Ag2CO38.0×10−12
The most soluble and least soluble compound are respectivelyAAgCl and PbCrO4BAgl and Ag2CO3CAgCl and Ag2CO3DAg2CO3 and AglThe KSP for Cr(OH)3 is 1.6×10−30. The molar solubility of this compound in water is
A2√1.6×10−30B4√1.6×10−30C4√1.6×10−3027D1.6×10−30/27
The solubility of CaF2(Ksp=3.4×10−11) in 0.1M solution of NaF would be
View SolutionThe Ksp of PbCrO4 is 1.0×10−16 . Then the molar solubility of PbCrO4 is
View SolutionThe Ksp for Mn(OH)2is1.6×10−13. What is the molar solubility of this compound in water?
View SolutionThe Ksp for Cr(OH)3 is 1.6×10−30. The solubility of this compound in water is :
View SolutionThe solubility of CuBr is 2×10−4 at 25∘C. The Ksp value for CuBr is
View Solution