A mixture that can be separated by sublimation is
A
AgCl+NaCl
B
BaCl2+NaCl
C
HgCl2+NaCl
D
MgCl2+NaCl.
Video Solution
Text Solution
The correct Answer is:C
HgCl2 sublimes on heating while NaCl donot.
|
Similar Questions
Knowledge Check
Identify the mixture in which the constituents cannot be separated by sublimation.
ANaphthalene+SandBIodine +IronCAmmonium chloride +SandDSulphur+sandIdentify the mixture in which the constituents cannot be separated by sublimation.
ANaphthalene+sandBIodine+IronCAmmonium chloride + sandDSulphur+SandAssertion: The process of conversion of water vapour into its liquid form is called Reason condensation.
Reason: A mixture of camphor and common salt can be separated by sublimation.AIf both assertion and reason are true and reason is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf assertion is false and reason is true.
Identify the mixture that can be separated by the diagram shown below.
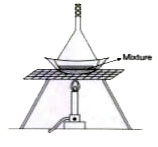
View SolutionIdentify the mixture that can be separated by the diagram shown below
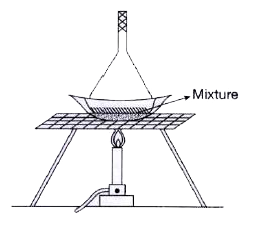
View SolutionWhich can be purified by sublimation ?
View SolutionIdentify the mixture which can be separated by magnetic separated by magnetic separation method.
View SolutionAssertion : A mixture of camphor and ammonium chloride cannot be separated by sublimation.
Reason : Camphor on heating sublimes, ammonium chloride does not.
View Solution

