Volume of H2 gas occupied by its one equivalent at S.T.P. is
A
22.4L
B
11.2L
C
5.6L
D
1.0L
Video Solution
Text Solution
The correct Answer is:B
1 equivalent of H2 = 1 g hydrogen
=11.2L at S.T.P.
|
Similar Questions
Knowledge Check
Assuming that O2 molecule is spherical in shape with radiusw 2∘A, the percentage of the volume of O2 molecules to the total volume of gas at S.T.P. is :
A0.0009B0.009C9.0E-5D0.00045The volume occupied by 0.2 mole of methane at N.T.P. is
A4.48dm3B8.96dm3C4.4dm3D2.24dm3Following is the graphical presentation of volumes occupied by different gases at S.T.P.
Which is/are not placed at correct position?
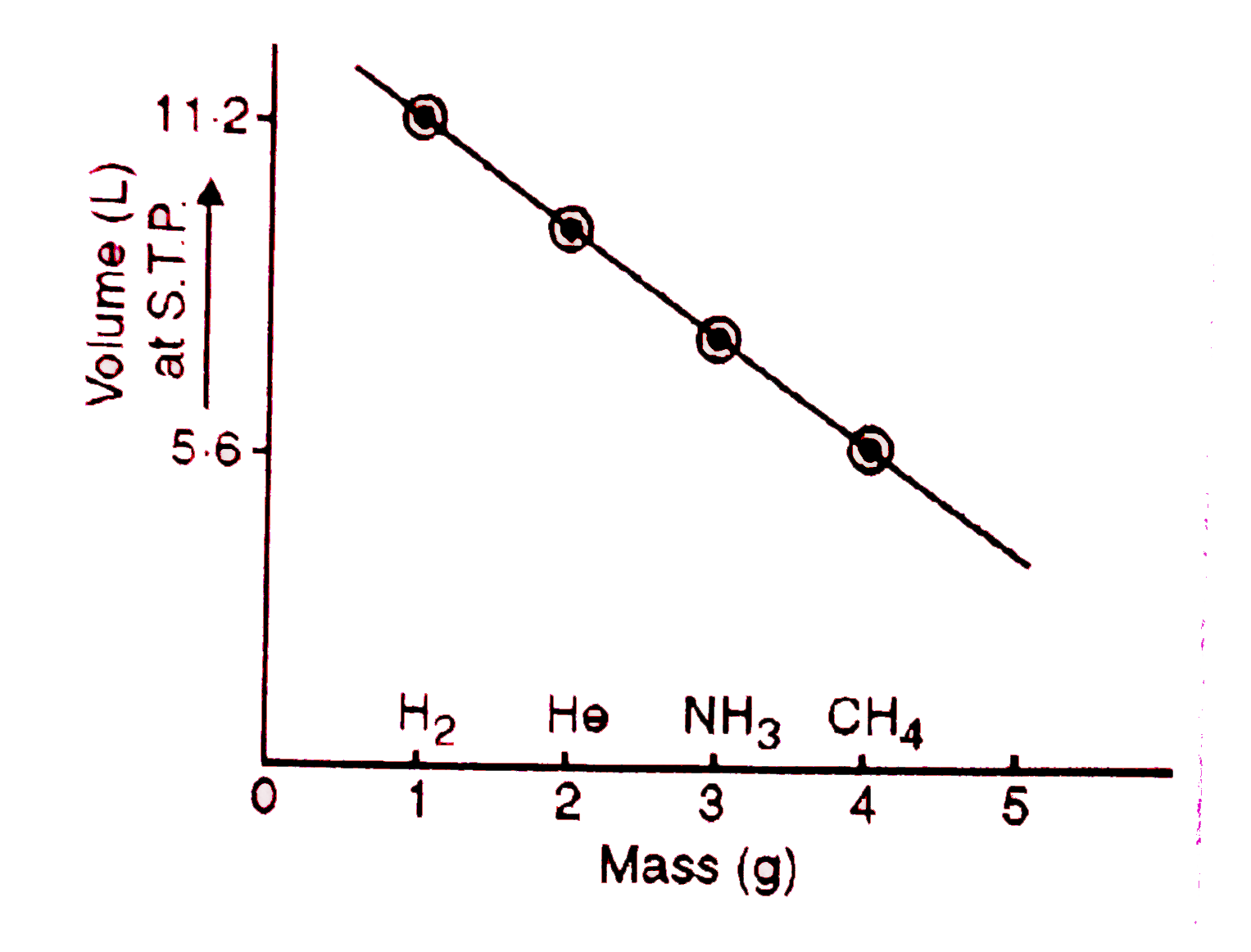 AH2,HeBHe,NH3CNH3,CH4DCH4,H2
AH2,HeBHe,NH3CNH3,CH4DCH4,H2
The volume of CO2 gas liberated at S.T.P by heating 5 grams of pure lime stone is
View Solution10 litres of O2 gas is reacted with 30 litres of CO at S.T.P. The volumes of each gas present at the end of the reaction are
View SolutionThe vapour density of a gas is 11.2. The volume occupied by 11.2 gms of the gas at S.T.P is
View SolutionAssertion : One mole of oxygen gas occupies 22.4 L volume at STP.
Reason : Volume of a gas depends on temperature and pressure and also on nature of gas.
View SolutionAt a given temperature, 1 mole O2 occupy 20dm3 of
volume. Thes volume occupied by 1 equivalent of
O2 is
View Solution
