sp3d2 hybrid orbitals are
A
linear
B
pentagonal bipyramidal
C
trigonal bipyramidal
D
octahedral
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for sp^3d^2 hybrid orbitals are by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Select the correct option of the following statements.
(P) Energy of an sp2 hybrid orbital is equal to that of an sp3 hybrid orbital.
(Q) % s character in each sp3d2 hybrid orbitals are at 90∘ to one another.
(S) Bond order of C-O bond in CO2−3 is 43ATTTTBFFTFCFFFTDFFTTThe geometry of sp3d2 hybrid orbitals is
AtetrahedralBpantagonal bipyramidalCtrigonal bipyramidalDoctahederalOnly sp and sp2 hybrid orbitals are involved in the formation of :
ACH3−CH=CH2BCH3−CH3CCH3−C≡CHDH2C=C=CH2
sp3 hybrid orbitals have equal s and p characters .
View SolutionTotal number of sp2 hybrid orbitals present in the following compound ?
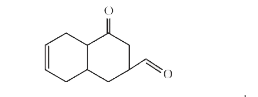
View Solution" Inter mixing of one s-orbital,three p-orbitals and one d-orbital to give "5sp^(3)d" hybrid orbitals is called "
View SolutionAssertion: sp3 hybrid carbon atom is more electronegative than sp hybrid carbon atom.
Reason: sp3 hybrid orbitals are more closer to the nucleus.
View SolutionNumber of sp2 hybrid orbitals in Benzene is
View Solution
