Consider the dipole moments of NH3andNF3. Which of the following is correct?
A
Dipole moment of NF3 is equal to that of NH3
B
Dipole moment of NH3 is equal in magnitude but opposite in sign to that of NH3
C
Dipole moment of NF3 is more than that of NH3
D
Dipole moment of NH3 is more than that of NF3
Video Solution
Text Solution
The correct Answer is:D
Both NH3andNF3 have pyramidal structures.
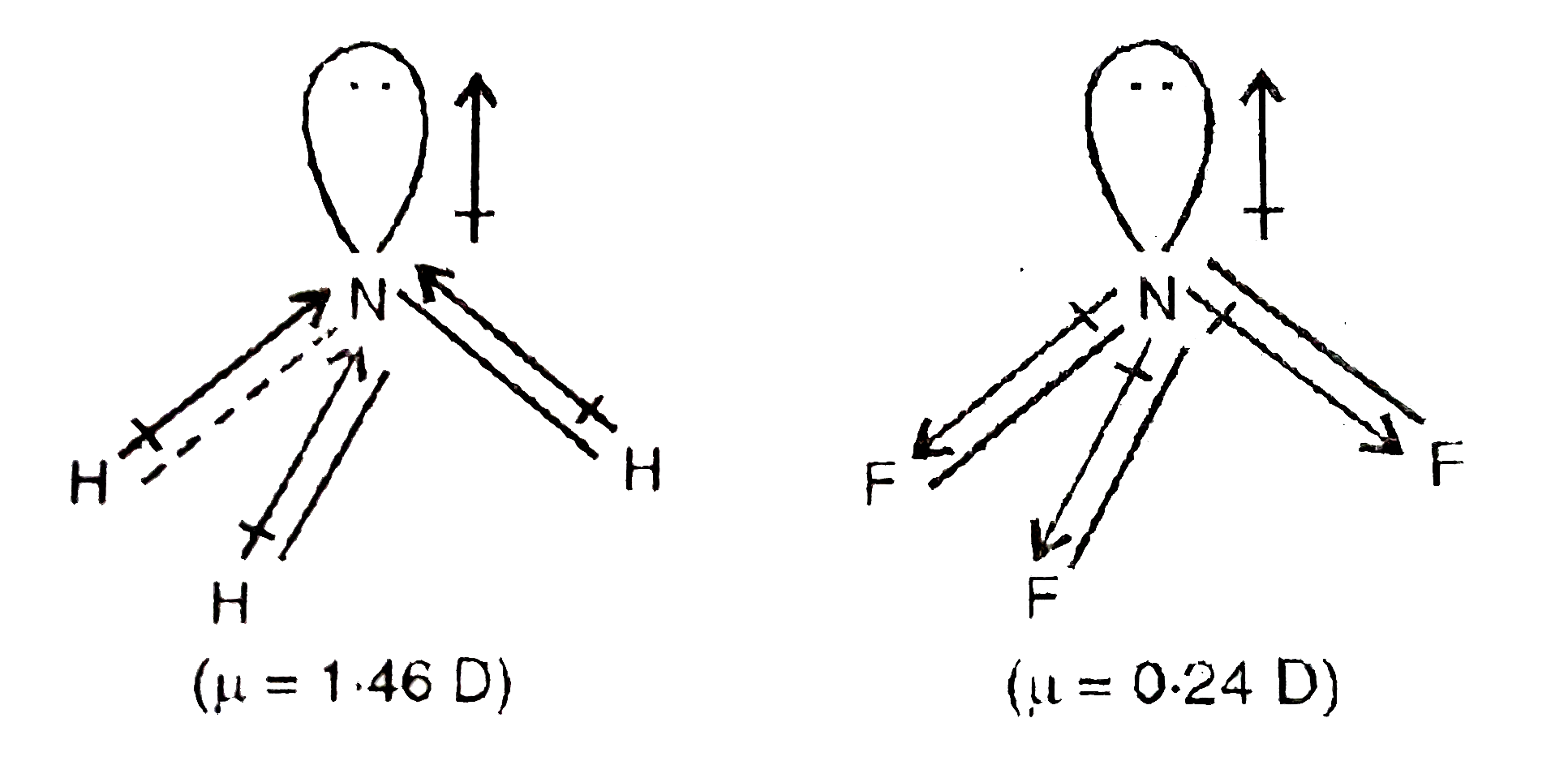
Electronegativity of N, H and F is in the order F gt N gt H. Due to lone pair and polarity of N-H and N-F bonds the dipole moment of NH3 is much more than that of NF3.
|
Similar Questions
Knowledge Check
The dipole moments of NH3NF3 and H2O are in the order.
ANH3<NF3<HOBNH3<NF3=H2OCNF3<NH3<H2ODNF3<H2O<NH3Consider the following carbonyl compounds
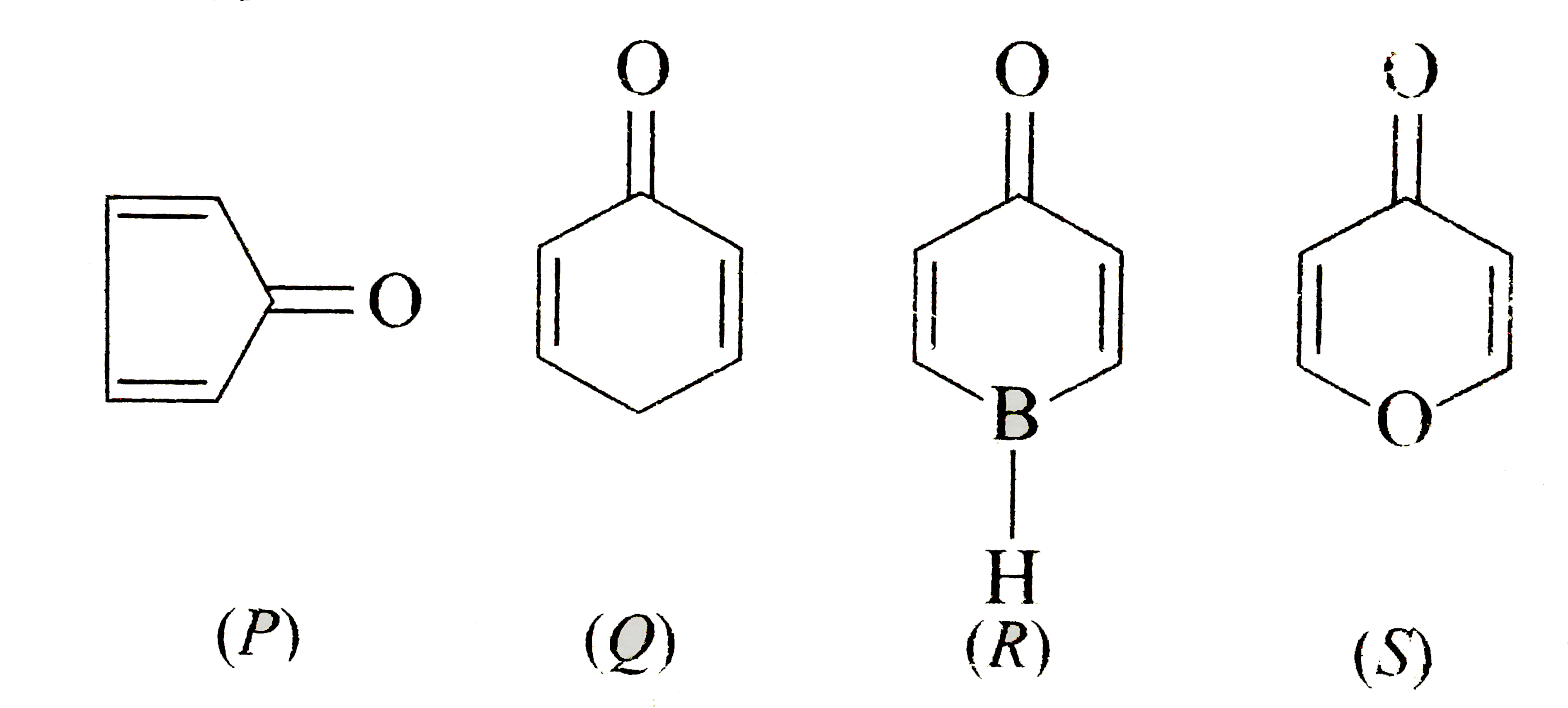
Which of the following is correct decreasing order of their dipole moment ?AP>R>Q>SBS>R>Q>PCS>Q>R>PDQ>S>R>PCorrect dipole moment order is
 Ap>qrBr>q>pCq>r>pDp>r>q
Ap>qrBr>q>pCq>r>pDp>r>q
NH3 and NF3, both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. Why does the dipole moment of NH3 is greater than that of NF3?
Choose the correct option.
View SolutionThe dipole moments of NH3,NF3andH2O are in the order :
View SolutionThe dipoles moment of NF3 is less than NH3 because
View SolutionStatement : The dipole moment of NH3 is less than NF3 .
Explanation : The lone pair preesent on N shows additive nature to N−H vector whereas it is subtractive to N−F vectro .
View SolutionThe dipoles moment of NF3 is less than NH3 because
View Solution