Which of the following molecule has highest dipole moment?
A
BF3
B
NH3
C
NF3
D
B2H6
Video Solution
Text Solution
The correct Answer is:B
BF3 and B2H6, are nonpolar, while NH3 and ..NF3 are polar to the pyramidal structures. In NH3, . the bond moments of three N-H bonds reinforces the dipole moment of lone pair but in NF3, the dipole moment of N-F bonds is opposite to that of lone pair as shown below:
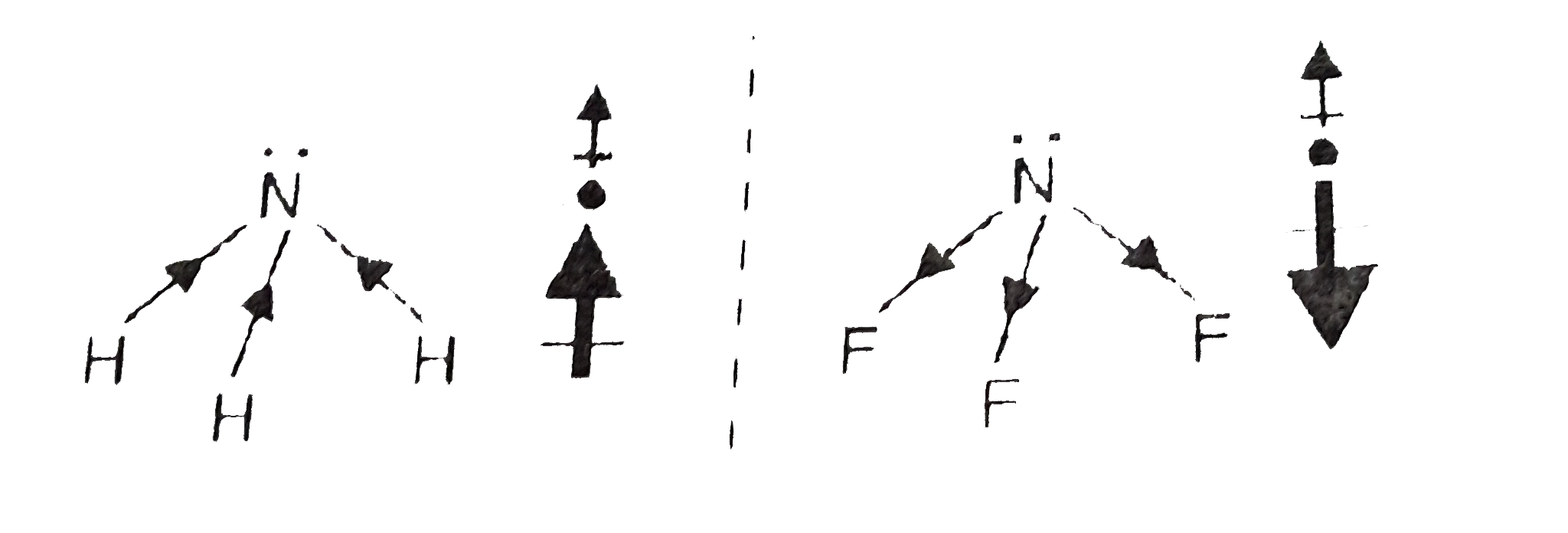
|
Similar Questions
Knowledge Check
Which of the following molecule has highest dipole moment?
ABF3BNH3CNF3DB2H6Which of the following molecule has highest dipole moment?
ABF3BNH3CNF3DB2H6Which of the following molecules has highest dipole moment ?
ACH3ClBCH2Cl2CCHCl3DCCl4
Which of the following molecule has highest dipole moment?
View SolutionWhich of the following molecule has net dipole moment?
View SolutionWhich of the following molecule has net dipole moment?
View SolutionWhich of the following molecule has a net dipole moment?
View SolutionWhich of the following molecules has zero dipole moment ?
View Solution

