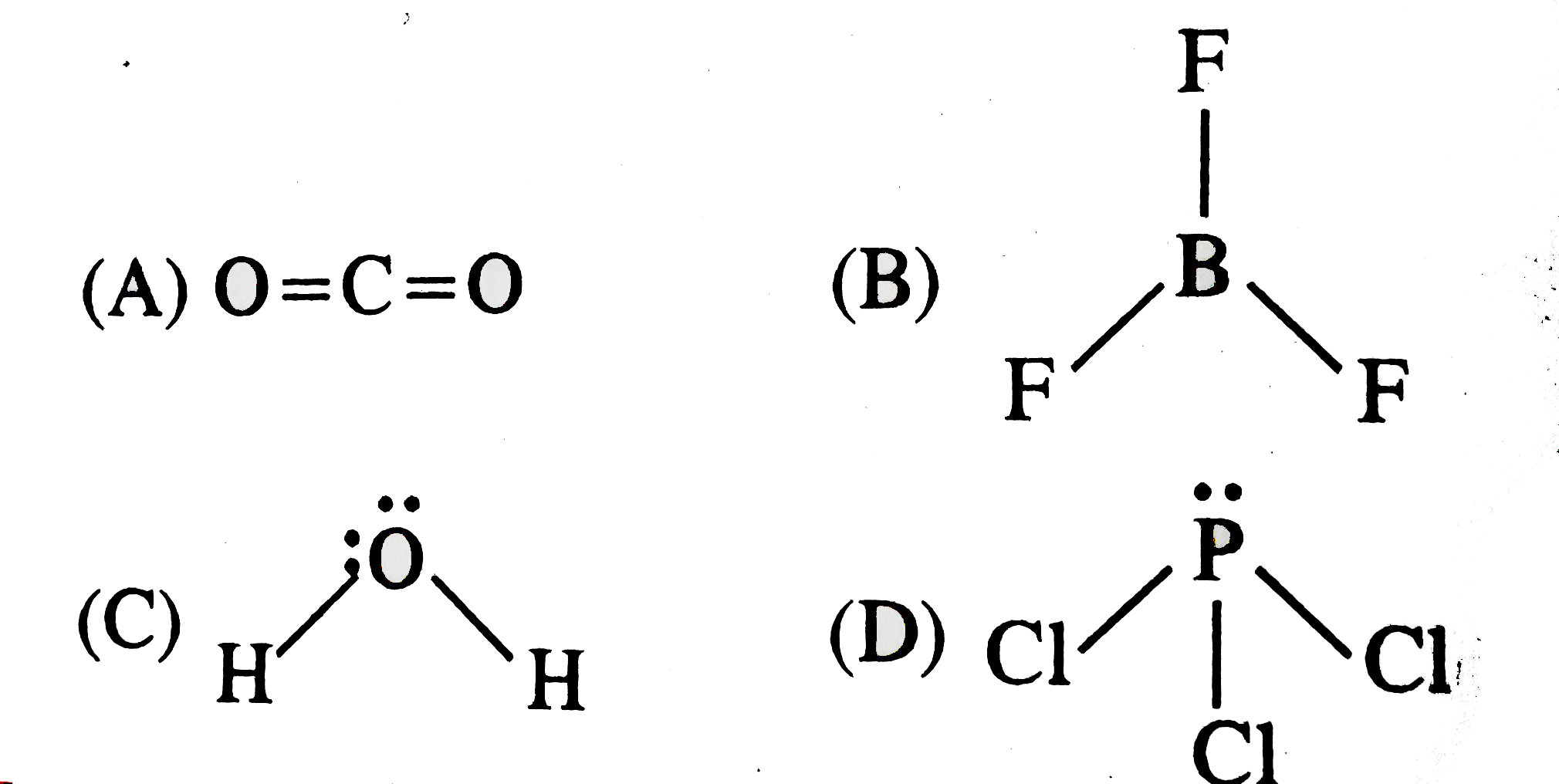
In which of the following molecules the central atom does not follow the octet rule ?
A
CO2
B
BF3
C
H2O
D
H2S
Video Solution
Text Solution
The correct Answer is:B
In BF3 the central atom (boron) has six electrons in the valence shell.
|
Similar Questions
Knowledge Check
In which of the following molecules the central atom does not follow the octel rule?
AH2OBNH3CH2DBH3In which of the following molecules does the central atom not follow the octet rule?
ACO2BBF3CH2ODPCl3In which one of the following compounds does the central atom obey the octet rule ?
AXeF4BXeOF2CSCl2DAlCl3
In which on the following, molecules central atom involves expansion of octet?
View SolutionIn which of the following molecule central atom is having complete octet
View SolutionIn which of the following molecules, the central atom does not have sp3 hybridization ?
View SolutionIn which of the following molecules the central atom does not have sp3 hybridization ?
View SolutionIn which of the following molecules the central atom does not have sp3 hybridisation?
View Solution

