The hybridization of oxygen atom in H2O2 is
A
sp3d
B
sp
C
sp2
D
sp3
Video Solution
Text Solution
The correct Answer is:D
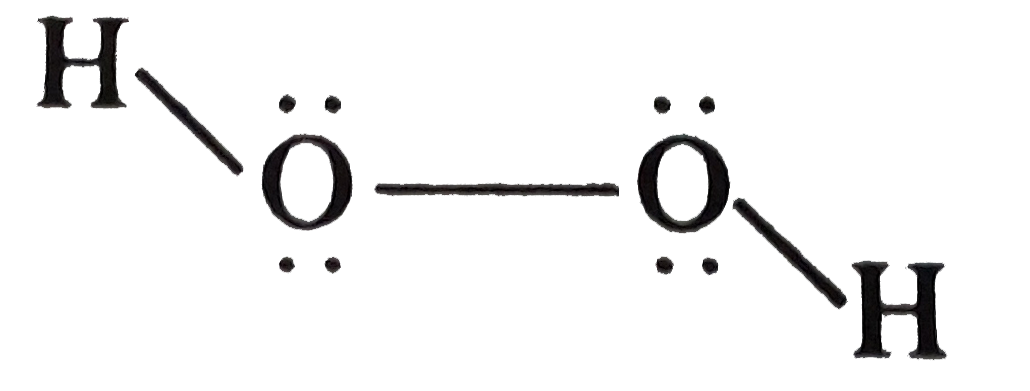
Each O atom has two bond pairs and two lone pairs. Hybridization of each O-atom is sp3.
|
Similar Questions
Knowledge Check
The hybridization of oxygen atom in H2O2 is
Asp3dBspCsp2Dsp3The hybridization of oxygen atom in H2O2 is
Asp3dBspCsp2Dsp3The hybridisation of oxygen in H2O is
Asp2Bsp3CspDNone of these
Oxidation state of oxygen in H2O2 is
View SolutionAmong the following, mention the correct hybridization of an oxygen atom in furan.
View SolutionThe oxidation number of oxygen in H2O2is
View SolutionOxidation number of oxygen in H2O2 is:
View SolutionOxidation satate of oxygen in H2O2 is:
View Solution
