Hyperconjugation involves
A
Delocalization of σ-electrons into an adjacent π-bond
B
Delocalization of n-electrons into an adjacent double bond
C
Delocalization of π-electrons into an adjacent π-bond
D
All are true.
Video Solution
Text Solution
The correct Answer is:A
Hyperconjugation involves delocalisation of α electrons into an adjacent π-bond.
|
Similar Questions
Knowledge Check
Hyperconjugation involes overlap of the _____.
Aσ−σ− bondsBσ bond -p orbitalCP−P orbitalsDπ−π bondsHyperconjugation is
Aσ−π conjugationBnoticed due to delocalisation of σandπ bondCno bond resonanceDall the aboveThe species which will not show hyperconjugation is
ACH3C+H2B(CH3)3CC+H2CCH3CH2C+H2DCH3C+HCH2
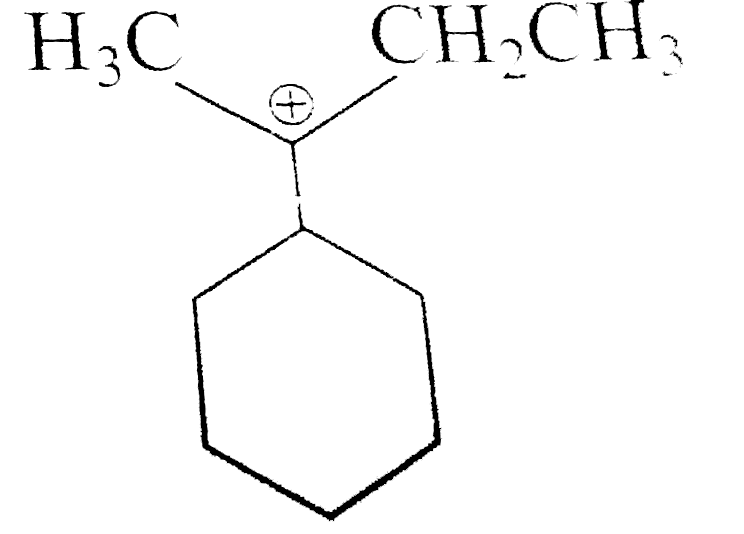
The total number of contributing structures showing hyperconjugation (involving C−H bonds) for the following carbocation is:
View SolutionHyperconjugation in Alkenes|| Stability OF Alkenes|| Heat OF Hydrogenation|| Heat OF Combustion||Hyperconjugation in Carbocation and Stability Comparison||Hyperconjugation in Free Radicals and stability comparison
View SolutionApplications of Hyperconjugation and Reverse Hyperconjugation
View SolutionHyperconjugation is possible in :
View SolutionHyperconjugation observed in
View Solution