How many monochlorobutanes will be obtained on chlorination of n-butane?
1
2
3
4
The correct Answer is:B
To determine how many monochlorobutanes can be obtained from the chlorination of n-butane, we can follow these steps:
Step 1: Understand the Structure of n-Butane
n-Butane has the molecular formula C4H10 and its structure can be represented as:
CH3−CH2−CH2−CH3
Step 2: Identify the Hydrogen Atoms
In n-butane, there are:
- 3 terminal hydrogen atoms (attached to the two CH3 groups)
- 6 hydrogen atoms attached to the three CH2 groups
Step 3: Chlorination Process
During chlorination, one hydrogen atom is replaced by a chlorine atom (Cl). This can occur at different positions in the n-butane molecule.
Step 4: Determine Possible Monochlorobutane Products
1. Chlorination at Terminal Carbons (CH3 groups):
- If we replace one of the terminal hydrogen atoms from the CH3 group, we will get:
CH2Cl−CH2−CH2−CH3
- This product is called 1-chlorobutane.
2. Chlorination at Internal Carbons (CH2 groups):
- If we replace one of the hydrogen atoms from the first CH2 group, we will get:
CH3−CHCl−CH2−CH3
- This product is called 2-chlorobutane.
Step 5: Identify Unique Products
- The chlorination at the terminal CH3 groups gives the same product regardless of which terminal hydrogen is replaced.
- The chlorination at the internal CH2 groups also gives the same product regardless of which internal hydrogen is replaced.
Conclusion
Thus, the two unique monochlorobutane products obtained from the chlorination of n-butane are:
1. 1-chlorobutane
2. 2-chlorobutane
Therefore, the total number of monochlorobutanes obtained is 2.
Final Answer
2 monochlorobutanes will be obtained on chlorination of n-butane.
---
n-Butane has the molecular formula C4H10 and its structure can be represented as:
CH3−CH2−CH2−CH3
In n-butane, there are:
- 3 terminal hydrogen atoms (attached to the two CH3 groups)
- 6 hydrogen atoms attached to the three CH2 groups
During chlorination, one hydrogen atom is replaced by a chlorine atom (Cl). This can occur at different positions in the n-butane molecule.
1. Chlorination at Terminal Carbons (CH3 groups):
- If we replace one of the terminal hydrogen atoms from the CH3 group, we will get:
CH2Cl−CH2−CH2−CH3
- This product is called 1-chlorobutane.
- If we replace one of the hydrogen atoms from the first CH2 group, we will get:
CH3−CHCl−CH2−CH3
- This product is called 2-chlorobutane.
- The chlorination at the terminal CH3 groups gives the same product regardless of which terminal hydrogen is replaced.
- The chlorination at the internal CH2 groups also gives the same product regardless of which internal hydrogen is replaced.
Thus, the two unique monochlorobutane products obtained from the chlorination of n-butane are:
1. 1-chlorobutane
2. 2-chlorobutane
2 monochlorobutanes will be obtained on chlorination of n-butane.
Similar Questions
Knowledge Check
2-chlorobutane obtained by chlorination of butane will be .
Ameso -formBd-formCrecemic formDl-formChloral is obtained by chlorination of
AchloroformBethanolCpropanolDmethanolBromination of n-butane gives
A1-bromobutane as the major productB2-bromobutane as the major productCboth 1-bromo and 2-bromobutane with equal percentageDboth 1-bromo and 2-bromo products whose percentage depends on temperature
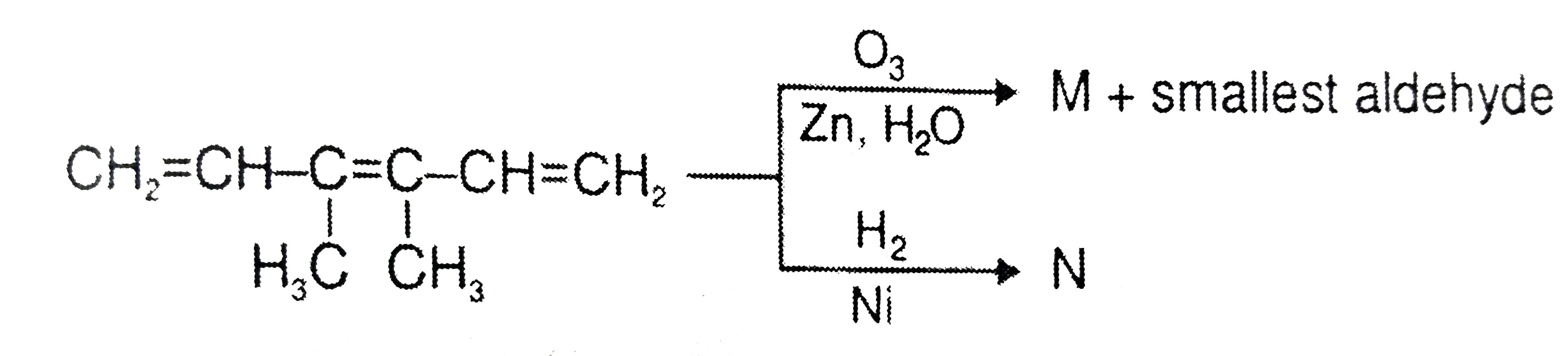
How many total monochloro structural isomers obtained on chlorination of product (N)
View SolutionButane cannot be obtained by-
View Solution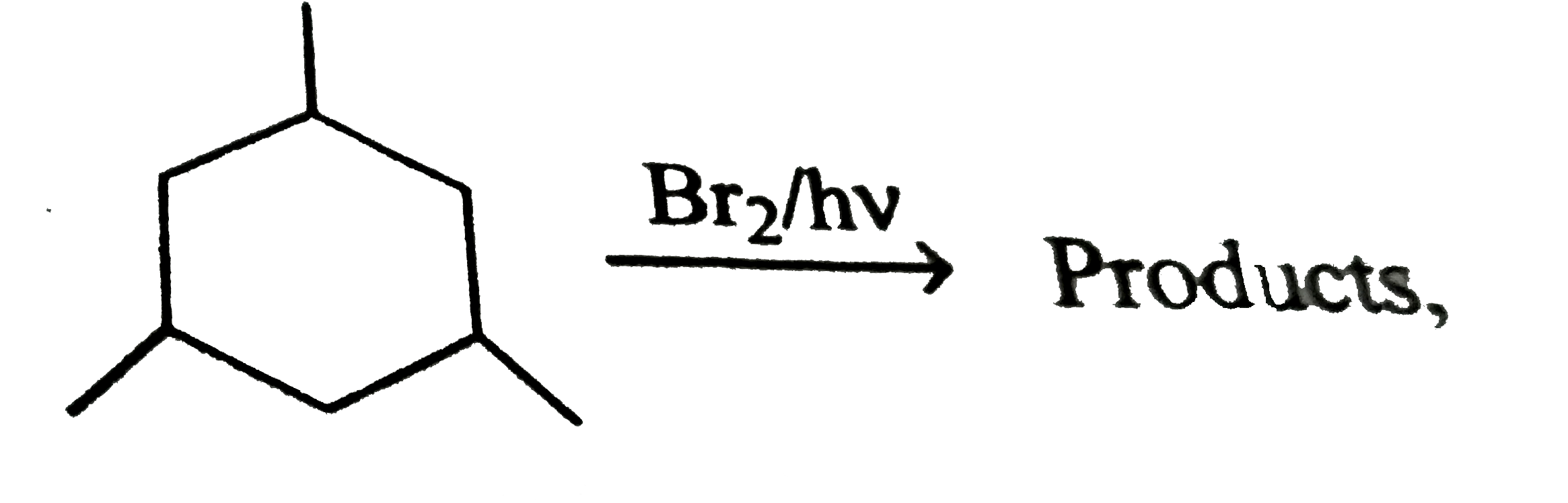
How many monobrominated products will be obtained by above reaction?
View SolutionHow many products will obtain in the following reaction ?
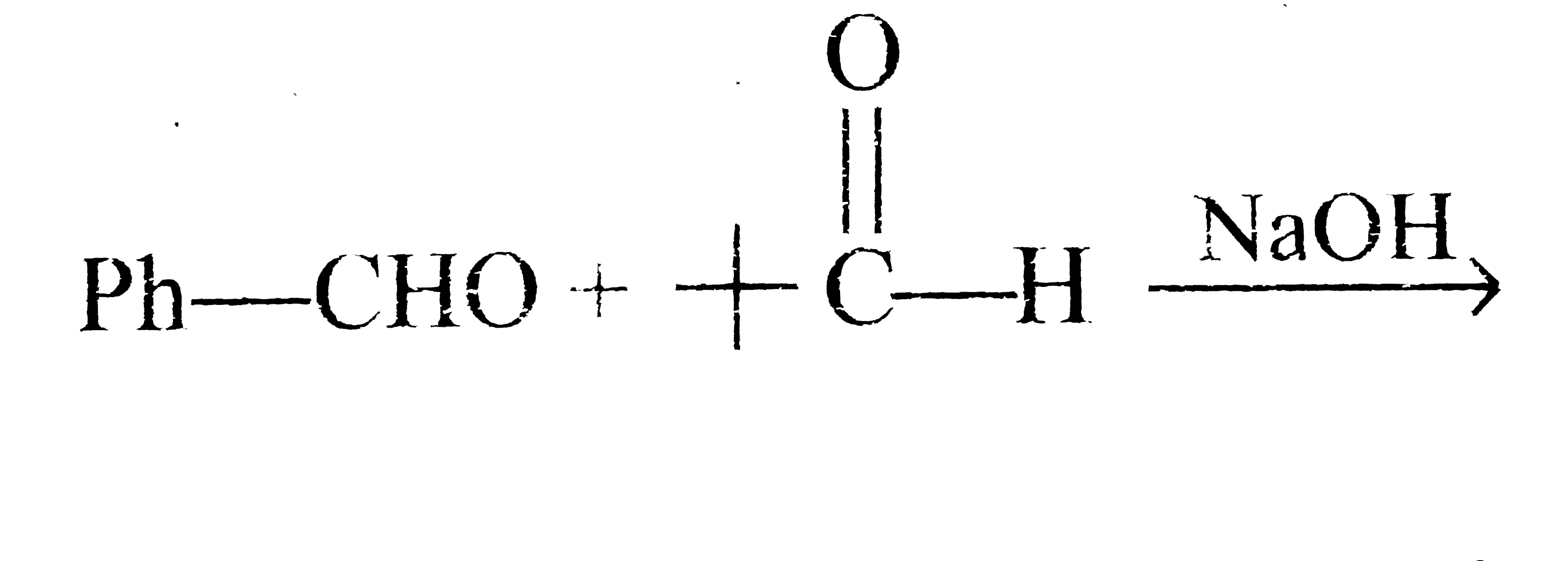
View SolutionHow many chiral compounds are possible on mono chlorination of 2-methyl butane ?
View Solution
