CaC2H2O−−→ARed tube hot−−−−−−−→BAlCl3−−−→CH3ClC
In this sequece B and C are.
benzene
Ethylbenzene
toluene
n-propylbenzene
The correct Answer is:C
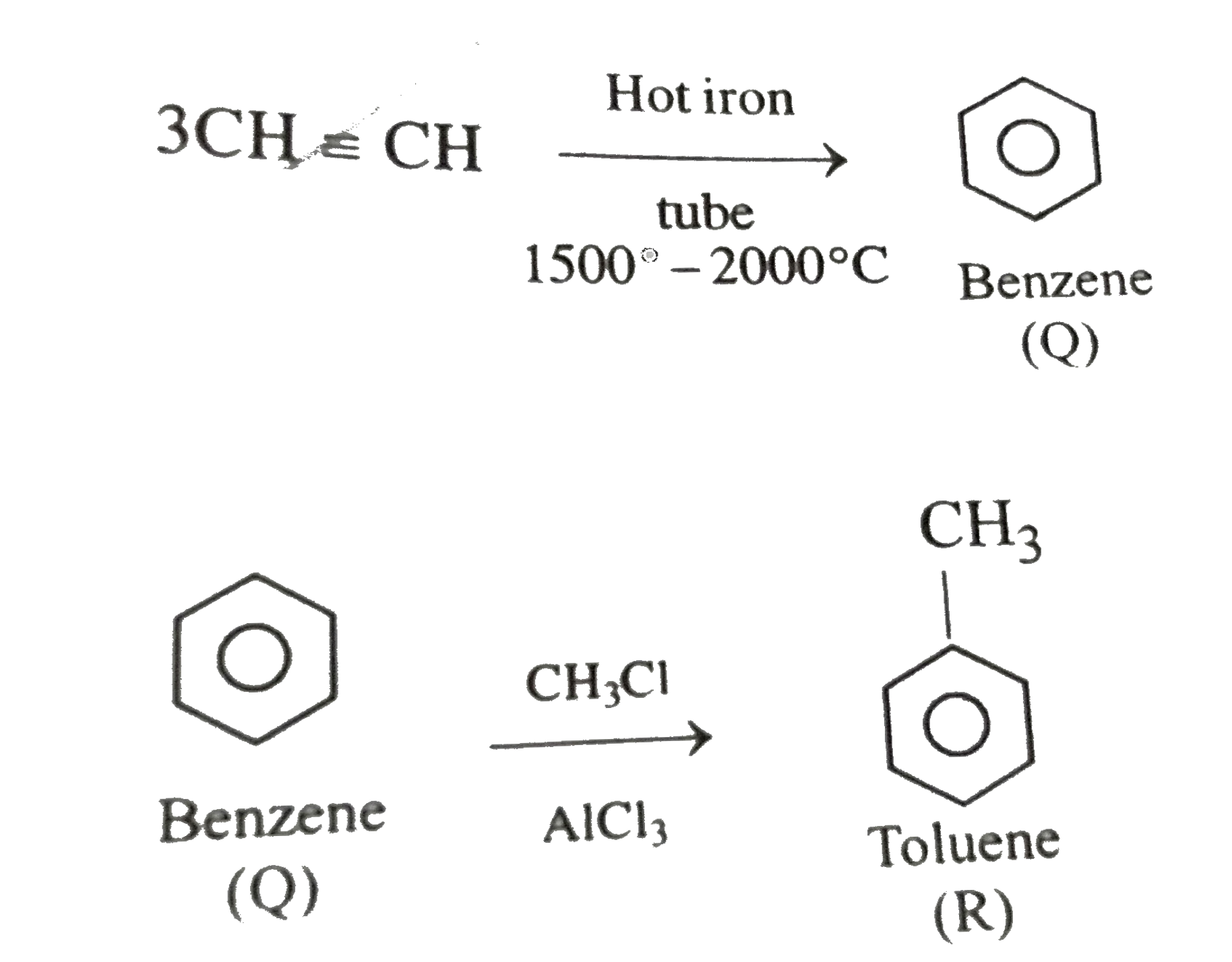
CaC2+2H2OCalcium carbide→HC≡CH+Ca(OH)2Acetylene(P)
Similar Questions
In the following sequence of reactions : Toluene KMnO4−−−−→ASOCl2−−−→BH2/Pd−−−→H2/PdC
View SolutionComplete the following reaction and name the product X, Y and Z. CaC_(2)overset(H_(2)O)rarrXoverset("hot metal tube")rarrYunderset(CH_(3)Cl)overset(AlCl_(3))rarrZ
View SolutionCaC_(2)overset(H_(2)O) to A overset("Red hot tube") to B underset(CH_(3)Cl) overset(AlCl_(3))to C , In this sequence B and C are
View SolutionIn the following sequence of reactions , CH_(3)-Broverset(KCN)toAoverset(H_(3)O^(+))toBunderset("ether")overset(LiAlH_(4))toC The end product ( C) is
View SolutionIn the following reaction, the product 'R' is:
CaC2H2O−−→Phot iron−−−−→tubeQCH3Cl−−−→AlCl3R
View SolutionGive the structures of A, B and C in the following: CH_(3)CHOunderset(H_(2)SO_(4))overset(K_(2)Cr_(2)O_(7))toAoverset(PCl_(5))toBunderset(AlCl_(3))overset(C_(6)H_(6))toC
View SolutionCaC2D2O−−→ARed hot−−−−→Cu tubeB,B is
View Solutionunderset("Calcium carbide")(CaC_(2))overset(H_(2)O)(to)(A)overset("Red hot Cu tube")(to)(B) , Product (B) of the reaction is:
View Solution

