Of the following compounds, which will have a zero dipole moment ?
A
m-Dinitrobenzene
B
trans-1,2-Dichloroethylene
C
cis-1,2-Dichloroethylene
D
1,4-Dichloroclohexane
Video Solution
Text Solution
The correct Answer is:B
In trans isomer, two electronegative chlorine atoms are opposite to one another, as such the resultant dipole moment is zero.
|
Similar Questions
Knowledge Check
Out of the following compounds which one will have zero dipole moment ?
AChloromethaneBDichloromethaneCTrichloromethaneDTetrachloromethaneWhich of the following compounds will have the highest dipole moment ?
A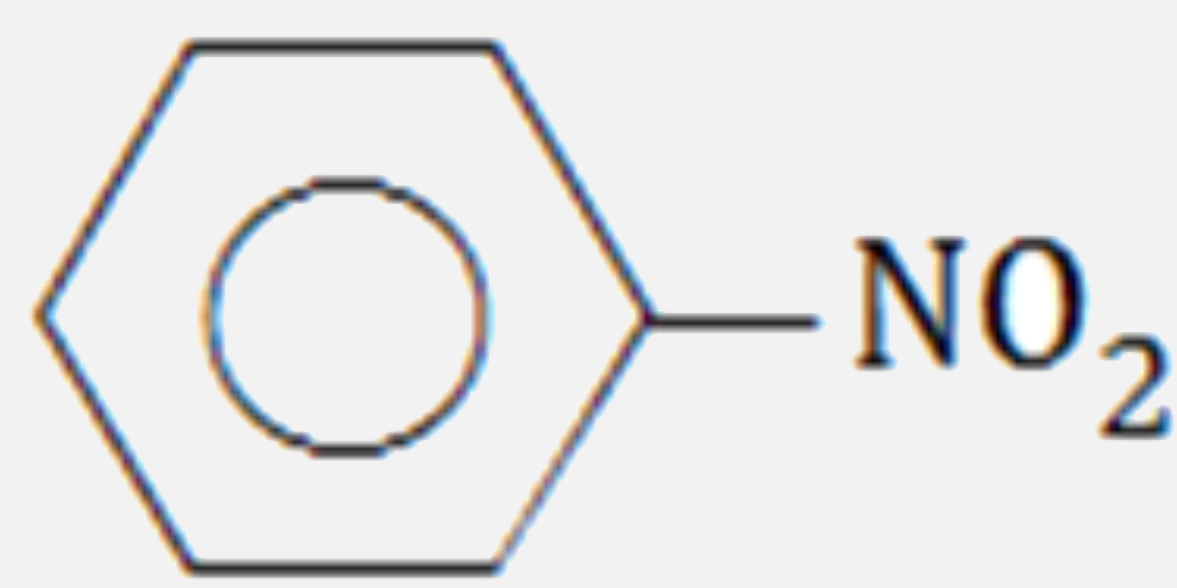 B
B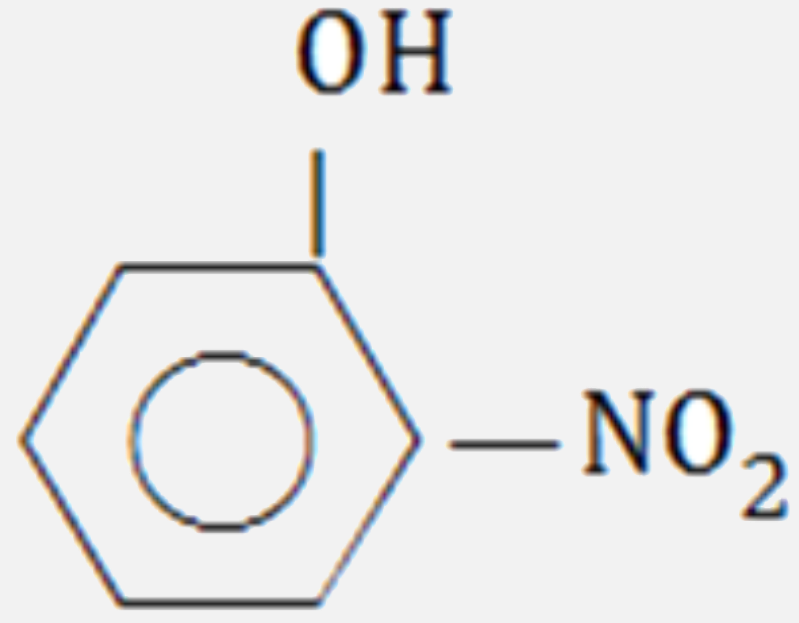 C
C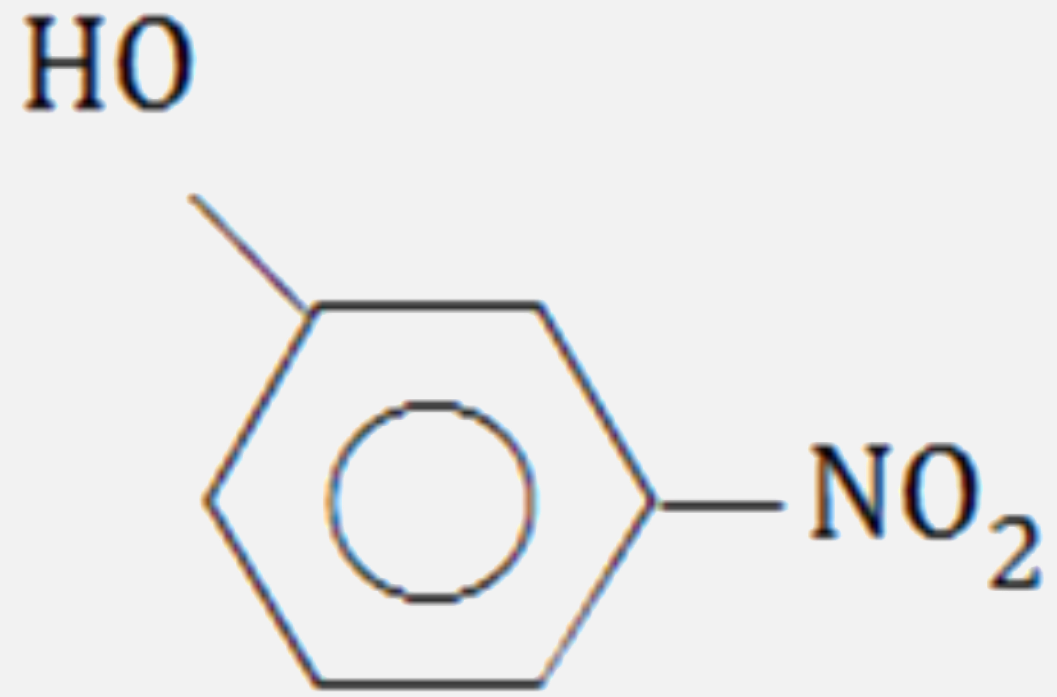 D
D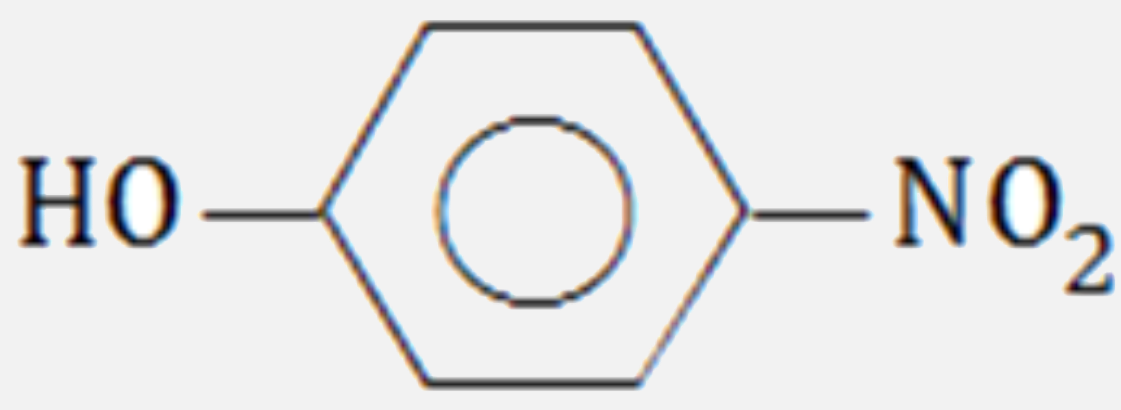
Which of the following set of molecules will have zero dipole moment ?
AAmmonia, beryllium difluoride, water, 1, 4-dichlorobenzeneBBoron trifluoride, hydrogen fluoride, carbon dioxide, 1, 3-dichlorobenzeneCNitrogen trifluoride, beryllium difluoride, water, 1, 3-dichlorobenzeneDBoron trifluoride, beryllium difluoride, carbon dioxide, 1, 4-dichlorobenzene
Which of the following set of molecules will have zero dipole moment?
View SolutionWhich of the following set of molecules will have zero diplole moment?
View SolutionWhich of the following molecules will have a peranent dipole moment ?
View SolutionWhich of the following compounds have non zero dipole moment.
View SolutionIn the following which one will have zero dipole moment ?
View Solution
