Which one of the following will have the maximum dipole moment
A
CH3F
B
CH3Cl
C
CH3Br
D
CH3I
Text Solution
The correct Answer is:B
CH3Cl has higher dipole moment than CH3F due to much longer C-Cl bond than C-F bond length.
|
Topper's Solved these Questions
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:Selected Straight Objective Type MCQs
Explore 78 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:Linked Comprehension Type MCQs
Explore 8 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:UNIT TEST - 5
Explore 37 Videos
- NO IDEABook:DINESH PUBLICATIONChapter:NO IDEAExercise:Unit Test -
Explore 1 Video
- POLYMERSBook:DINESH PUBLICATIONChapter:POLYMERSExercise:Matrix
Explore 5 Videos
Similar Questions
Knowledge Check
Which of the following will have maximum dipole moment?
A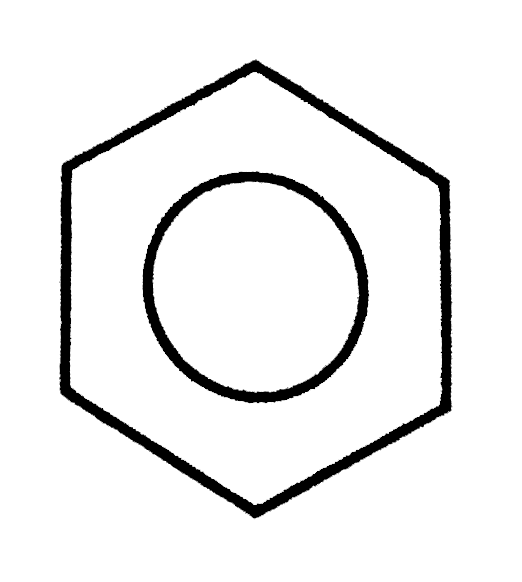 B
B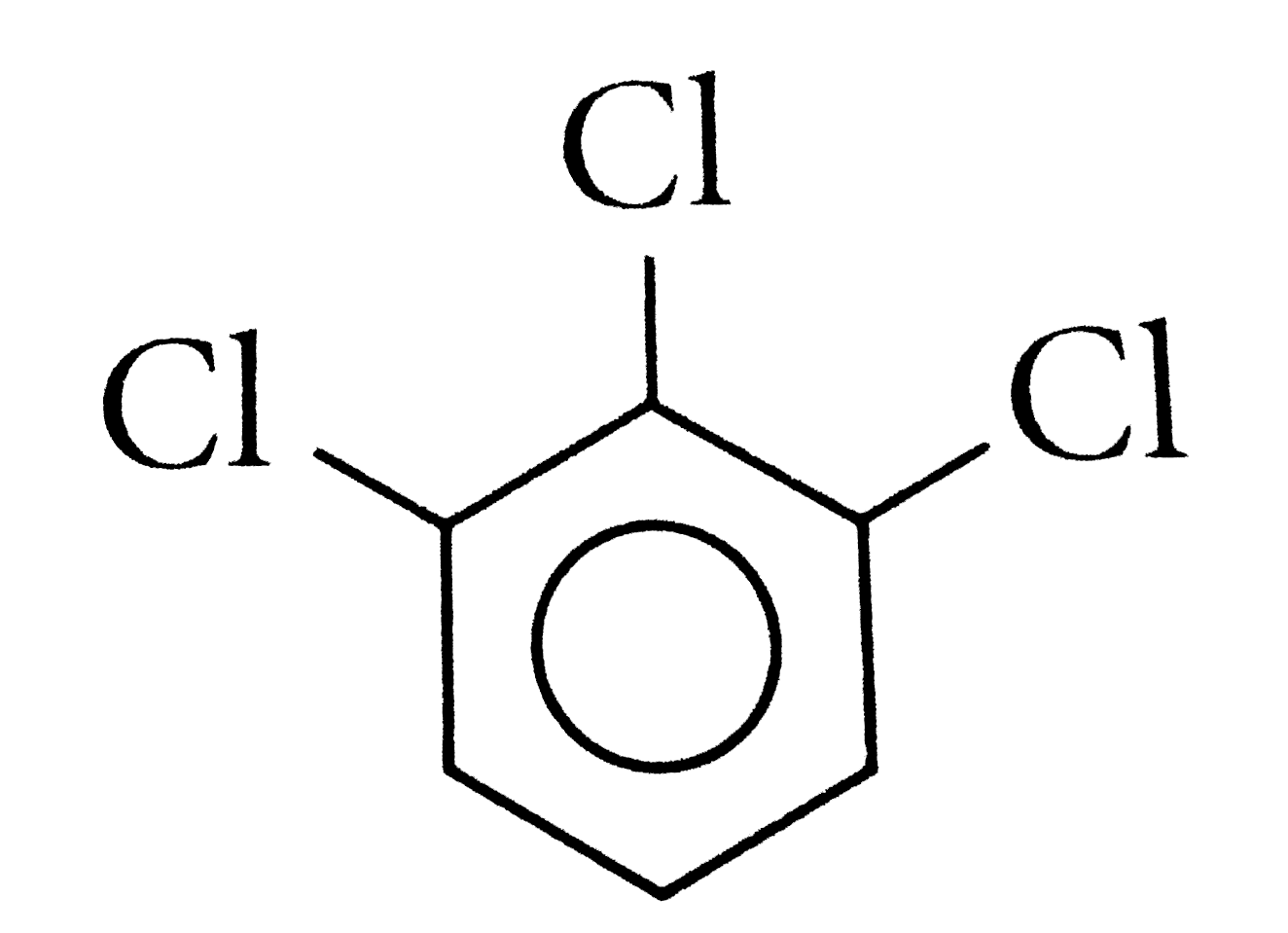 C
C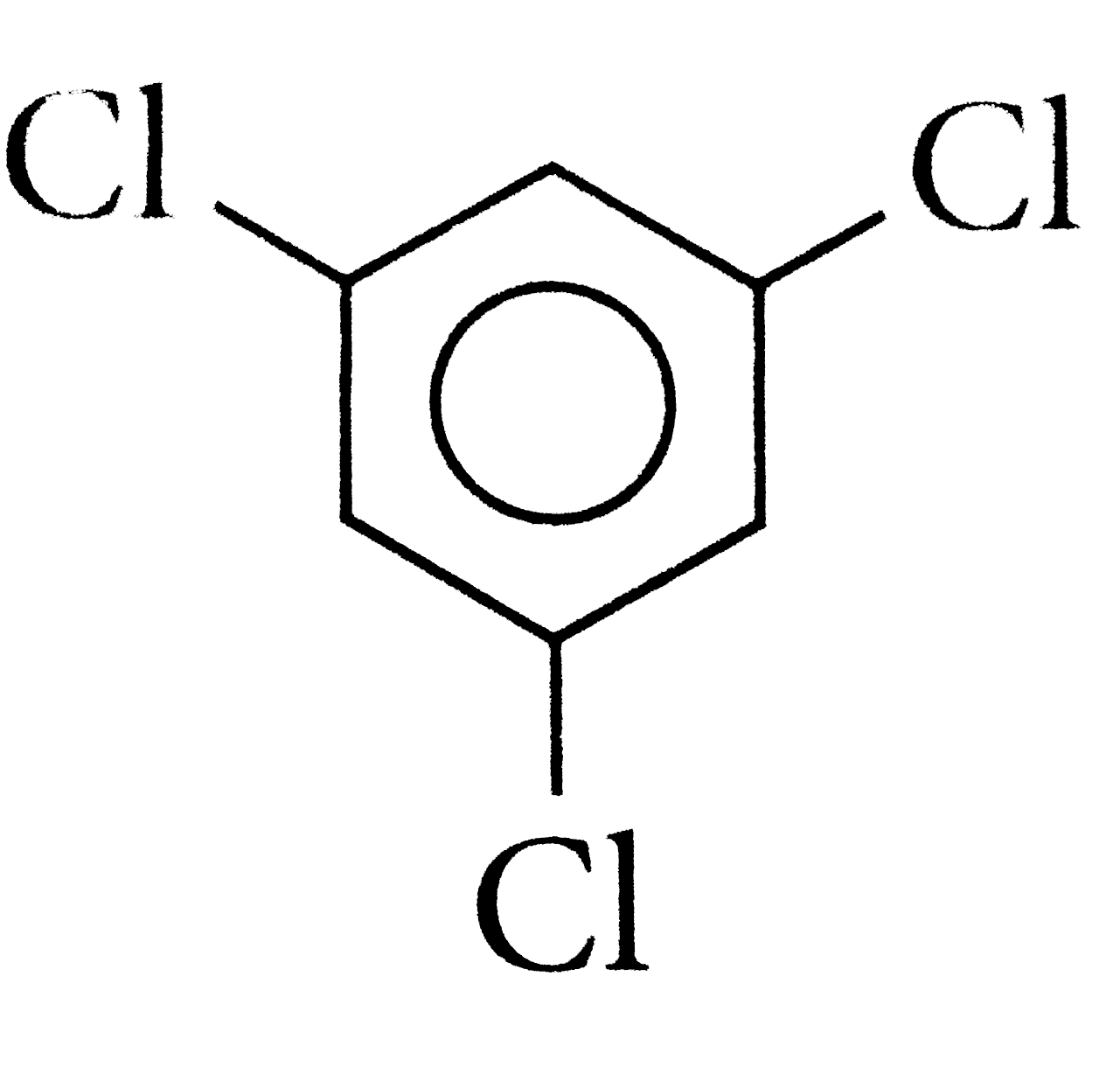 D
D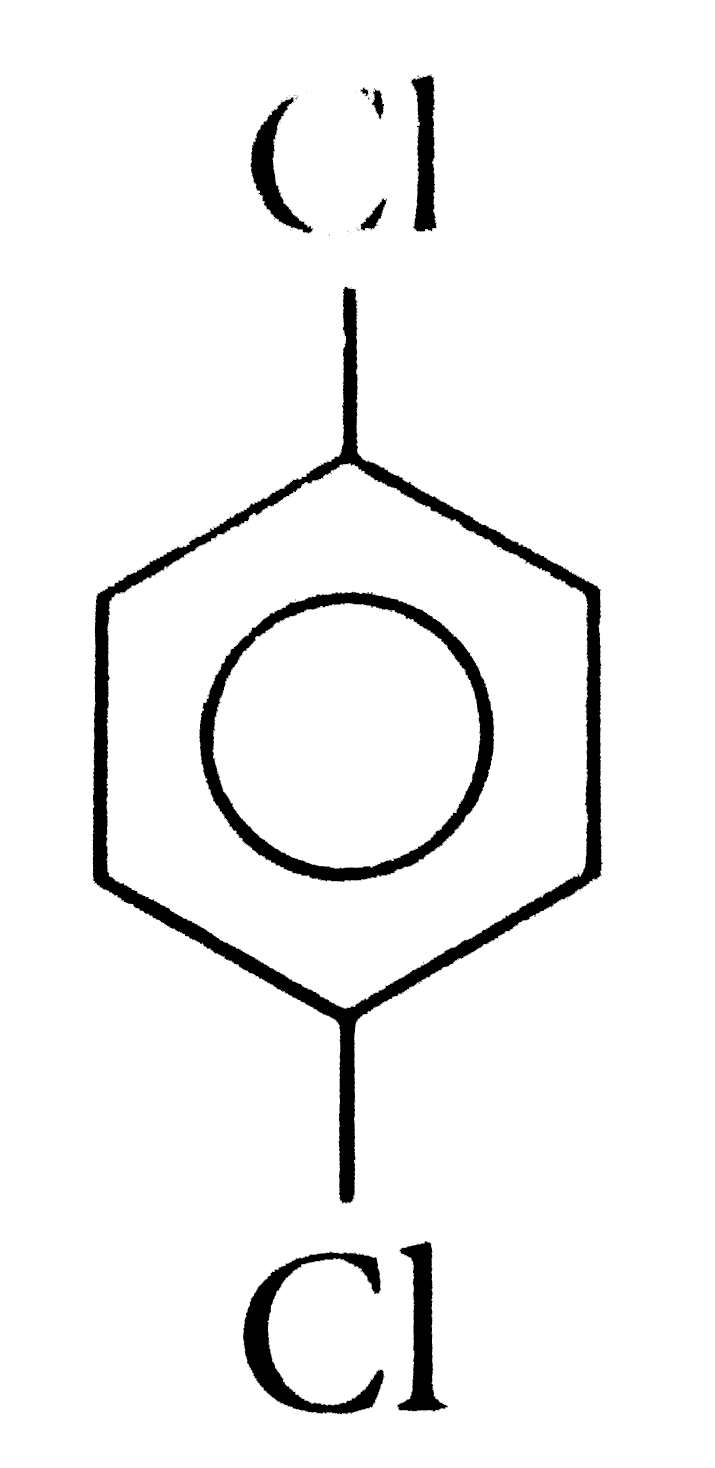
Which of the following will have maximum dipole moment?
A B
B C
C D
D
Which of the following molecules has the maximum dipole moment ?
ACO2BCH4CNH3DNF3
Which of the following molecules has the maximum dipole moment?
View SolutionWhich of the following molecules has the maximum dipole moment?
View SolutionWhich has the maximum dipole moment ?
View SolutionWhich of the following will have the highest dipole moment ?
View SolutionWhich of the following will have larger dipole moment ?
View Solution

