Which one of the following is not the correct order of boiling points of alkyl / aryl halides ?
A
CHCl3>CH2Cl2
B
CH3(CH2)3Cl>CH3(CH2)2Cl
C
(CH3)3CCl>(CH3)2CHCH2Cl
D
CH3(CH2)3Cl>CH3CH3CH2CHClCH3
Video Solution
Text Solution
The correct Answer is:C
As the number of halogens increases, boiling point increases. Further, boiling points decrease with braching. Hence (C ) is correct.
|
Topper's Solved these Questions
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:Selected Straight Objective Type MCQs
Explore 78 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:Linked Comprehension Type MCQs
Explore 8 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Book:DINESH PUBLICATIONChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUP CONTAINING HALOGENS Exercise:UNIT TEST - 5
Explore 37 Videos
- NO IDEABook:DINESH PUBLICATIONChapter:NO IDEAExercise:Unit Test -
Explore 1 Video
- POLYMERSBook:DINESH PUBLICATIONChapter:POLYMERSExercise:Matrix
Explore 5 Videos
Similar Questions
Knowledge Check
The correct order of boiling points is
ACH3F<CH3Cl<CH3Br<CH3IBCH3F<CH3I<CH3Br<CH3ClCCH3I<CH3Br<CH3Cl<CH3FDCH3F<CH3Br<CH3Cl<CH3ICorrect order of boiling point is
An-butane lt ethoxyethane lt pentan-1-olBn-butane ltpentan-1-olltethoxyethaneCEthoxyethane ltn-butane lt pentan-1-olDPentan-1-ol ltethoxyethane ltn-bulaneThe correct order of boiling point is :
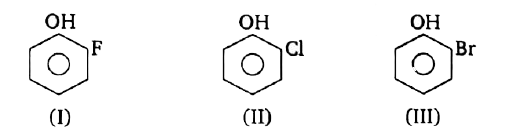 AI>II>IIIBIII>II>ICII>I>IIIDIII>I>II
AI>II>IIIBIII>II>ICII>I>IIIDIII>I>II
The correct order of boiling points of alkyl halides is
View SolutionWhich of the following is correct order of boiling point ?
View SolutionWhich one of the following is the correct decreasing order or boiling point ?
View SolutionThe correct order of boiling points of hydrogen halide is:
View SolutionThe decreasing order of boiling points of alkyl halides is
View Solution
