Among the following four structures I to IV
I CH3 ∣C2H5−CH−C3H7
II O CH3 || |CH3−C−CH−C2H5
III H−H∣C⊕∣H
IV CH3∣C2H5−CH−C2H5 it is true that
C2H5−CH3| C H−C3H7
CH3−O∣∣C−CH3| C H−C2H5
H−H∣C∣HO
C2H5−C−CH3| C H−C2H5
The correct Answer is:B
Chiral compounds have of least chiral centre. All four atoms or groups attached to carbon are different
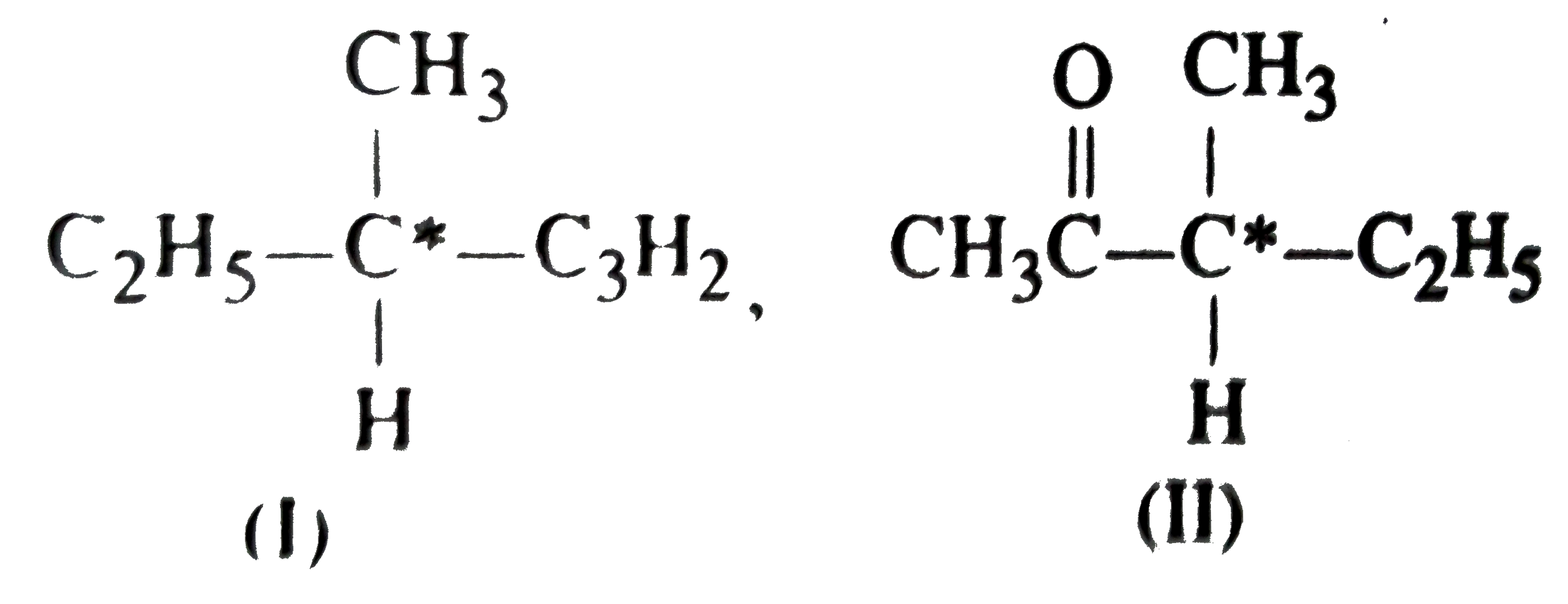
Similar Questions
Identify the saturated compounds : (i) CH_(3)CH_(2)CH=O (ii) C_(2)H_(5)CH=CH_(2) (iii) C_(2)H_(5)OH (iv) CH_(2)=CHCOOH
View SolutionFor the given four structures , I to IV, which of the following is correct ?
C2H5−CH3∣CH−C3H7(I) CH3−OPC−CH3∣CH−C2H5(II)CH3−OPC−C2H5∣CH−C2H5(III) C2H5−CH3∣CH−C2H5(IV)
View SolutionAmong the following four structures I to IV
(I) H5C2−CH3∣CH−C3H7
(II) H3C−O∣C−CH3∣CH−CH2H5
(III) H−H∣H+∣H
(IV) H5C2−CH3∣CH−C2H5
In is true that
View SolutionWhich of the following compounds will exhibit pro-cons isomerism. (i) (CH_(3))_(2)C = CH-C_(2)H_(5) (Ii) CH_(2) = CBr_(2) (iii) C_(6)H_(5)-CH=CH-CH_(3) (iv) CH_(3) -CH = C Cl-CH_(3)
View Solutionनिम्न अभिक्रियाओं पर विचार कीजिए
(i) (CH3)2CH−CH2BrC2H5OH−−−−→(CH3)2CH−CH2OC2H5+HBr
(ii) (CH3)2CH−CH2BrC2H5O−−−−−−→(CH3)2CH−CH2OC2H5+Br−
अभिक्रियाएँ (i) और (ii) की क्रियाविधियाँ क्रमशः हैं
View SolutionWhich of the following compounds will show cis-trans isomerism ?
(i) (CH3)2C=CH−C2H5
(ii) CH2=CBr2
(iii) C6H5CH=CHC6H5
(iv) CH3CH=CClCH3
View Solutionनिम्न चार संरचनाओं के लिए
C2H5−CH3∣CH−C3H7(I) CH3−O∣∣C−CH3∣CH−C2H5(II)
H−H∣C⊕∣H(III) C2H5−CH3∣CH−C2H5(IV)
सत्य कथन है
View SolutionRank the following free radicals in order of decreasing stability (I) C_(6)H_(5)CH_(6)H_(5) (II) C_(6)H_(5) -CH-CH=CH_(2) (III) CH_(3)-overset(.)CH - CH_(3) (IV) C_(6)H_(5)-overset(.)CH-CH_(3) (V) CH_(3)CH=CHCH_(2)overset(.)CH_(2) (VI) CH_(3)-CH_(2)-underset(CH_(3)) underset(|)overset(.)C-CH_(3)
View Solution

