The pKapKb values of some bases are as follows. Which is strongest base ?
A
pKb=9.4
B
pKb=3.32
C
pKa=4.0
D
pKa=11.1
Video Solution
Text Solution
The correct Answer is:B
Smaller is the pKb, more stronger the base and smaller is the pKa, more stronger is the acid.
|
Similar Questions
Knowledge Check
Maximum pKb value is of
A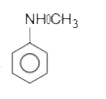 B(CH3CH2)2NHC(CH3)2NHD
B(CH3CH2)2NHC(CH3)2NHD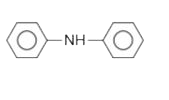
Maximum pKb value is of
A(CH3)2NHB(CH3CH2)2NHC D
D
pK4 values are given for some acids. Which is the strongest acid
A2.05B3.12C10.02D1.24
The base with lowest pKa value is
View SolutionSelect the pKa value of the strongest acid from the following :
View SolutionSelect the pKa value of the strongest acid from the following
View SolutionpKa values of four acids are given below at 25∘C . Indicate the strongest acid
View SolutionWhich has highest pKb value?
View Solution

