Nitrobenzene on reaction with HNO3/H2SO4 on 80∘−100∘C forms which one of the following products
A
1,4-Dinitrobenzene
B
1, 2, 4-Trinitrobenzene
C
1, 2-Dinitrobenzene.
D
1, 3-Dinitrobenzene.
Text Solution
The correct Answer is:D
NO2 is a m-directing group and hence 1, 3-dinitrobenzene is formed.
|
Similar Questions
Knowledge Check
Nitrobenzene on reaction with conc HNO3/H2SO4 at 80−100∘C forms which one of the following products .
A1,4-DinitrobenzeneB1,2,4-TrinitrobenzeneC1,2-DinitrobenzeneD1,3-DinitrobenzeneNitrobenzene on reaction with conc HNO3/H2SO4 at 80−100∘C forms which one of the following products .
A1,3-dinitrobenzeneB1,4-DinitrobenzeneC1,2,4-TrinitrobenzeneD1,2-DinitrobenzeneNitrobenzene on reaction with conc. HNO3/H2SO4 at 80−100∘C forms which one of the following products?
A1, 4-DinitrobenzeneB1, 2, 4-TrinitrobenzeneC1, 2-DinitrobenzeneD1,3-Dinitrobenzene
Nitrobenzene on reaction with conc. HNO3H2SO4 at 80−100∘C forms which one of the following products?
View SolutionNitrobenzene on reaction with conc. HNO3H2SO4 at 80−100∘C forms which one of the following products?
View SolutionIncreasing order of rate of reaction with HNO3/H2SO4 is
(i)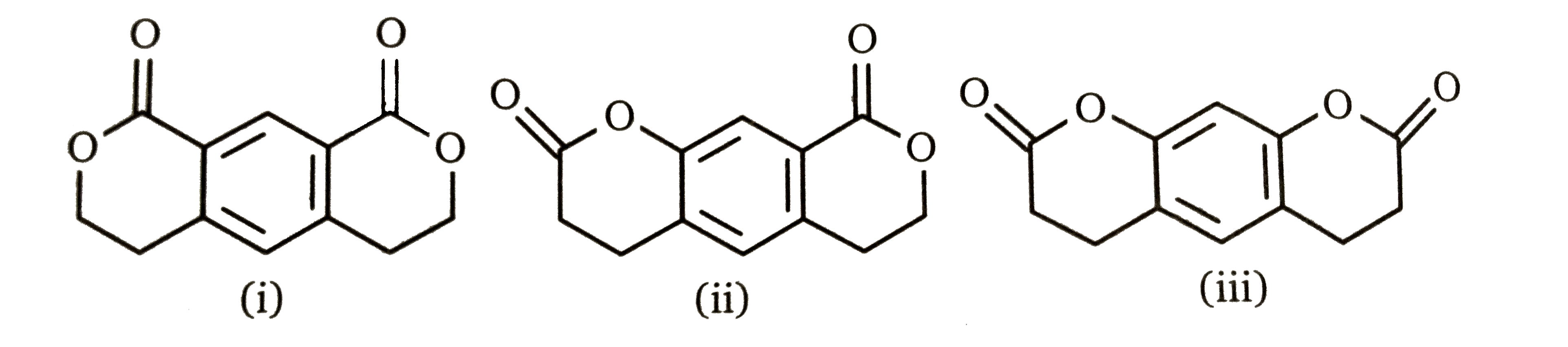
View SolutionBenzene on treating with a mixture of conc. HNO3andH2SO4 at 100∘C gives
View SolutionIncreasing order of rate of reaction with Conc. HNO3/H2SO4 is
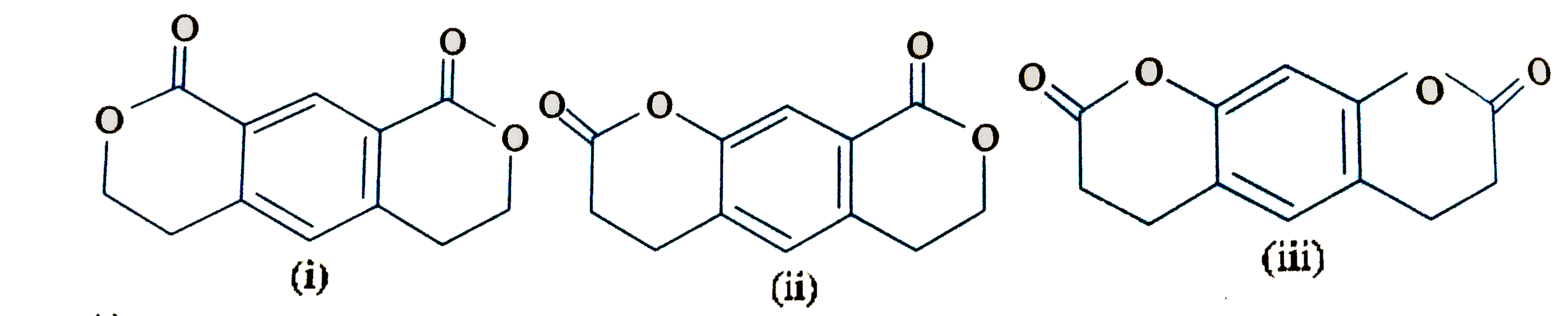
View Solution

