The strongest base among the following agent.
A
2, 4, 6-Trinitro-N, N-dimethylaniline
B
Aniline
C
2, 4, 6-Trinitroaniline
D
N, N-Dimethylaniline.
Text Solution
The correct Answer is:A

Presence of two bulky NO2 groups at o−positions to N-atom does not allow the lone pair on N to take part in resonance. This increases the availability of lone pair on nitrogen. Further, the presence of two methyl groups increase, the basic character due to +I effect of CH3. This makes 2, 4, 6-Trinitro−N, N-dimethyl aniline most basic, out of the given four choices
|
Similar Questions
Knowledge Check
The strongest base among the following is
A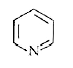 B
B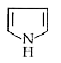 C
C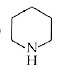 D
D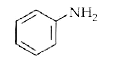
The strongest base among the following is
AC6H5NH2B(C6H5)2NHCC2H5NH2D(C2H5)2NHThe strongest base among the following is
ANaOHBKOHCLiOHDCsOH

