Which one of the following compounds has sp2 hybridization?
A
CO2
B
SO2
C
N2O
D
CO
Video Solution
Text Solution
The correct Answer is:B
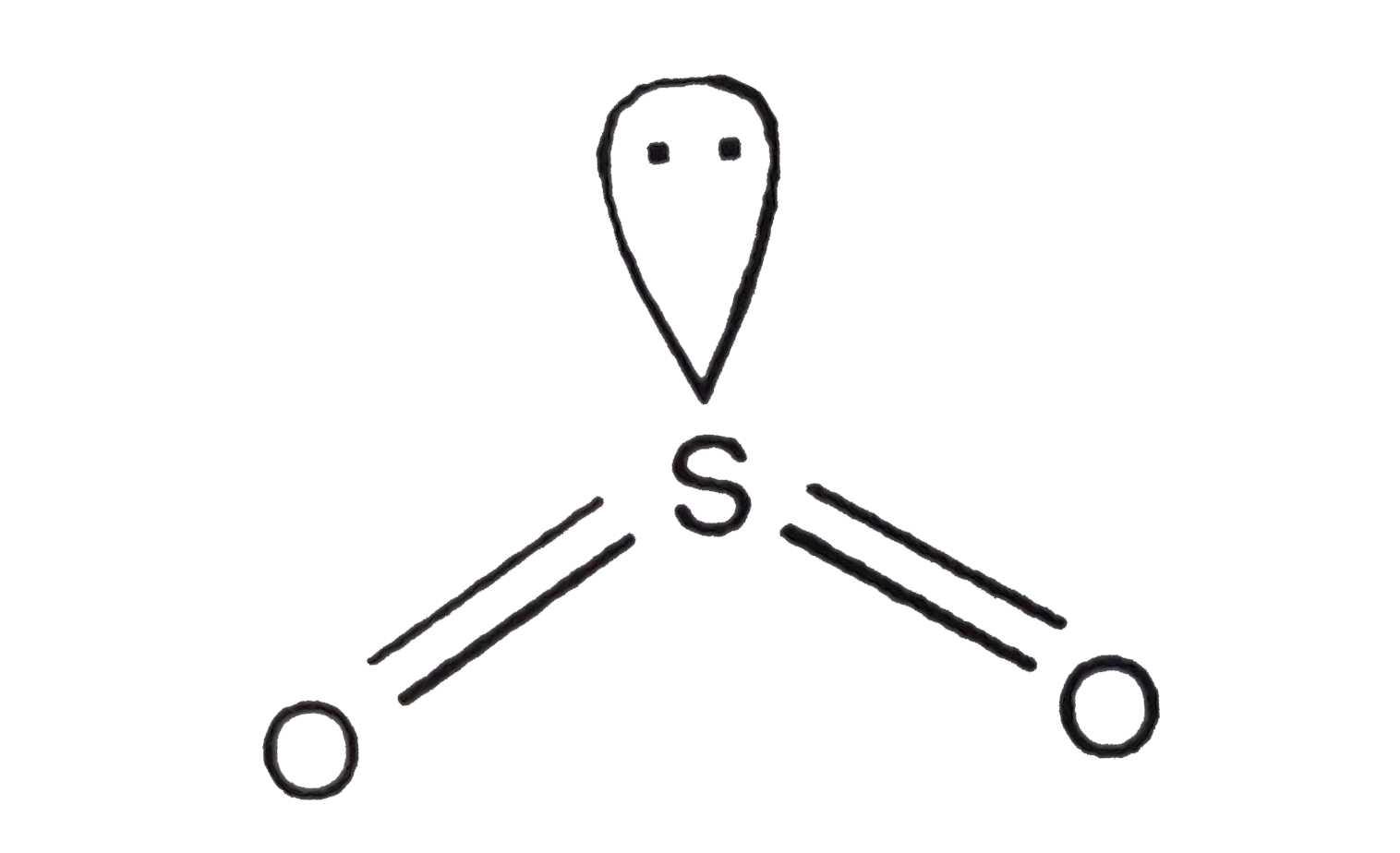
In SO2 , S is sp2 hybridised SO2 is a bent angular structure with a lone pair of electron on S.
In CO2,N2O,andCO,C,NandC are sp-hybridised and all of them have linear structure as shown below
O=C=O,N≡N→O,C←−−=O
|
Similar Questions
In which of the following compound sp2-hybridisation is absent
View SolutionWhich of the following compounds? sp^3d - Hybridized
View Solutionక్రింది వాటిలో SP3 సంకరీకరణం గల సమ్మేళనం
View Solutionनिम्नलिखित यौगिकों में से एक ध्रुवीय यौगिक है और उसका केन्द्रीय परमाणु sp2- संकरित अवस्था में है वह यौगिक है
View Solutionनिम्न में से कौन - सा यौगिक बायें से दायें जाने पर क्रमानुसार sp2−sp2−sp−sp संकरण प्रदर्शित करता है ?
View SolutionIn which of the compound sp2−sp2−sp−sp hybridization shift from left to right?
View SolutionIn which of the compound sp2−sp2−sp−sp hybridization shift from left to right?
View Solutionनिम्न यौगिकों में से किस एक यौगिक में केन्द्रीय परमाणु क संकरण sp2 है तथा वह यौगिक ध्रुवीय है?
View Solution

