Identify the correct order of acidic strength of CO2,CuO,CaO and H2O.
A
CaO<CuO<H2O<CO2
B
H2O<CuO<CaO<CO2
C
CaO<H2O<CuO<CO2
D
H2O<CO2<CaO<CuO
Video Solution
Text Solution
The correct Answer is:A
CuO and CaO are basic oxides (oxides of metals).Out of the two CaO is more basic.Therefore their acid strength is in the order CaO lt CuO.
CO2 and H2O are oxides of non-metals.
H2O is neutral whereas CO2 is acidic in nature, Therefore their acidic strength is in the order H2O<CO2.
Thus, correct order of acidic strength is CaO<CuO<H2O<CO2.
|
Similar Questions
Knowledge Check
Correct order of acidic strengths
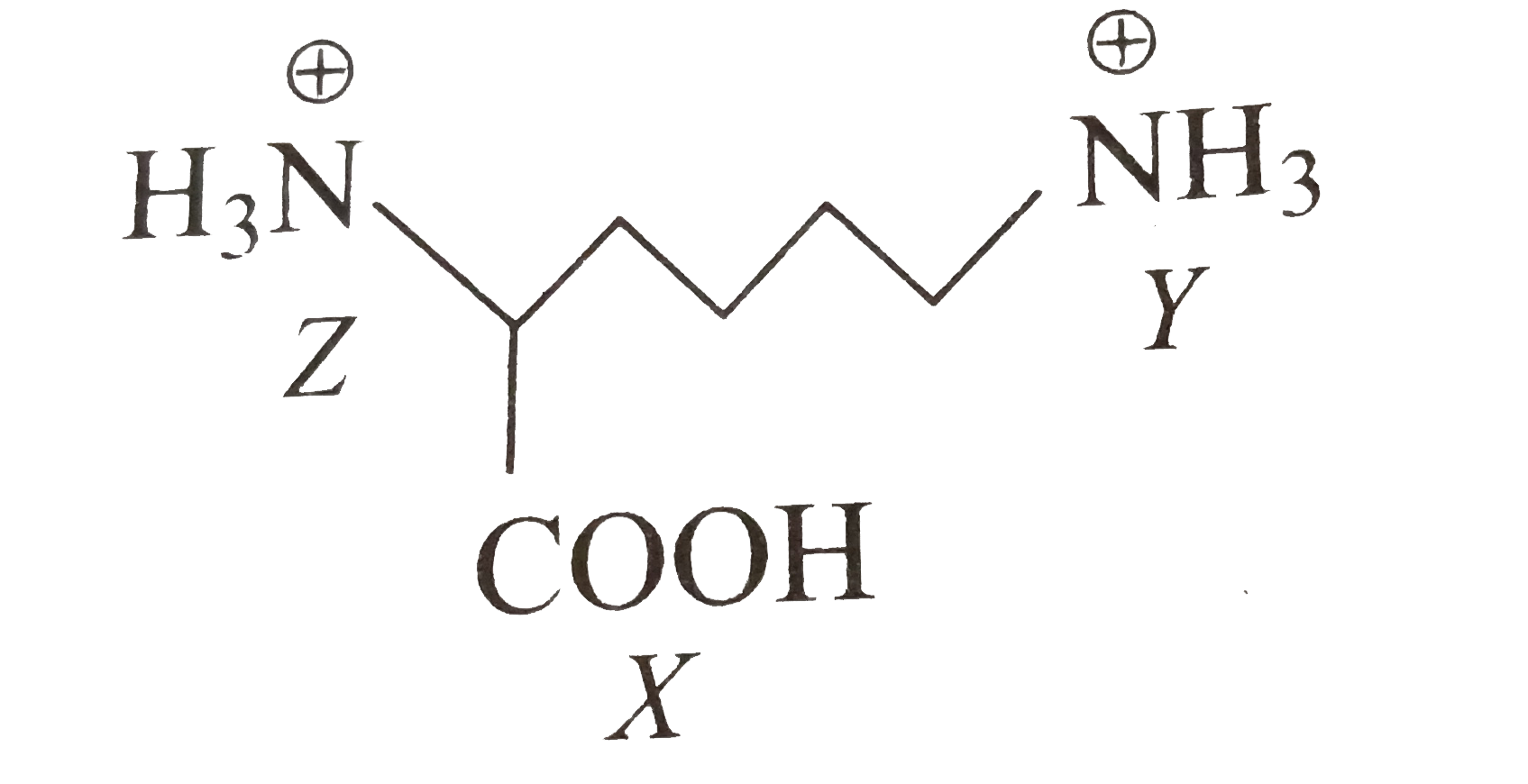 AX>Y>ZBZ>Y>XCY>Z>XDX>Z>Y
AX>Y>ZBZ>Y>XCY>Z>XDX>Z>YIdentify the correct order of acidic strengths of CO2,CuO,CaOandH2O
ACaO<CuO<H2O<CO2BH2O<CuO<CaO<CO2CCaO<H2O<CuO<CO2DH2O<CO2<CaO<CuOThe correct order of acidic strength is
AK2O>CaO>MgOBCO2>N2O5>SO3CNa2O>MgO>Al2O3DCl2O7>SO2>P4O10

