The correct IUPAC name of K2[Zn(OH)4] is
potassium tetrahydroxyzinc(II)
potassium tetrahydroxozincate(II)
potassium tetrahydroxizincate(IV)
potassium hydroxozinc(II)
The correct Answer is:B
To determine the correct IUPAC name of the coordination compound K2(Zn(OH)4), we will follow the systematic naming rules for coordination compounds. Here’s a step-by-step breakdown of the solution:
Step 1: Identify the Cation and Anion
The compound K2(Zn(OH)4) consists of:
- Cation: K+ (potassium)
- Anion: (Zn(OH)4)2−
Step 2: Name the Cation
The cation is potassium, and since there are two potassium ions, we simply write "potassium" in the name.
Step 3: Identify the Ligands
The anionic part (Zn(OH)4)2− contains hydroxide ions (OH−). Hydroxide is a negatively charged ligand, and since there are four hydroxide ligands, we use the prefix "tetra-" to indicate the number of ligands.
Step 4: Name the Ligands
The ligand derived from hydroxide is called "hydroxo." Since there are four hydroxide ligands, we write "tetrahydroxo."
Step 5: Name the Central Metal
The central metal ion is zinc. Since it is part of an anionic complex, we modify the name of zinc to "zincate."
Step 6: Determine the Oxidation State of the Metal
To find the oxidation state of zinc in the complex, we can set up the following equation based on the charges:
- Let the oxidation state of zinc be x.
- The total charge contributed by the four hydroxide ligands is −4 (since each hydroxide has a charge of -1).
- The overall charge of the anionic complex is −2.
Thus, we have:
x−4=−2
Solving for x:
x=+2
Step 7: Combine All Parts
Now we can combine all parts to form the complete name:
- Cation: potassium
- Ligands: tetrahydroxo
- Central metal: zincate
- Oxidation state: (II)
Putting it all together, the IUPAC name is:
Potassium tetrahydroxozincate(II)
Final Answer
The correct IUPAC name of K2(Zn(OH)4) is Potassium tetrahydroxozincate(II).
---
The compound K2(Zn(OH)4) consists of:
- Cation: K+ (potassium)
- Anion: (Zn(OH)4)2−
The cation is potassium, and since there are two potassium ions, we simply write "potassium" in the name.
The anionic part (Zn(OH)4)2− contains hydroxide ions (OH−). Hydroxide is a negatively charged ligand, and since there are four hydroxide ligands, we use the prefix "tetra-" to indicate the number of ligands.
The ligand derived from hydroxide is called "hydroxo." Since there are four hydroxide ligands, we write "tetrahydroxo."
The central metal ion is zinc. Since it is part of an anionic complex, we modify the name of zinc to "zincate."
To find the oxidation state of zinc in the complex, we can set up the following equation based on the charges:
- Let the oxidation state of zinc be x.
- The total charge contributed by the four hydroxide ligands is −4 (since each hydroxide has a charge of -1).
- The overall charge of the anionic complex is −2.
x−4=−2
Solving for x:
x=+2
Now we can combine all parts to form the complete name:
- Cation: potassium
- Ligands: tetrahydroxo
- Central metal: zincate
- Oxidation state: (II)
Potassium tetrahydroxozincate(II)
The correct IUPAC name of K2(Zn(OH)4) is Potassium tetrahydroxozincate(II).
Similar Questions
Knowledge Check
The correct IUPAC name of
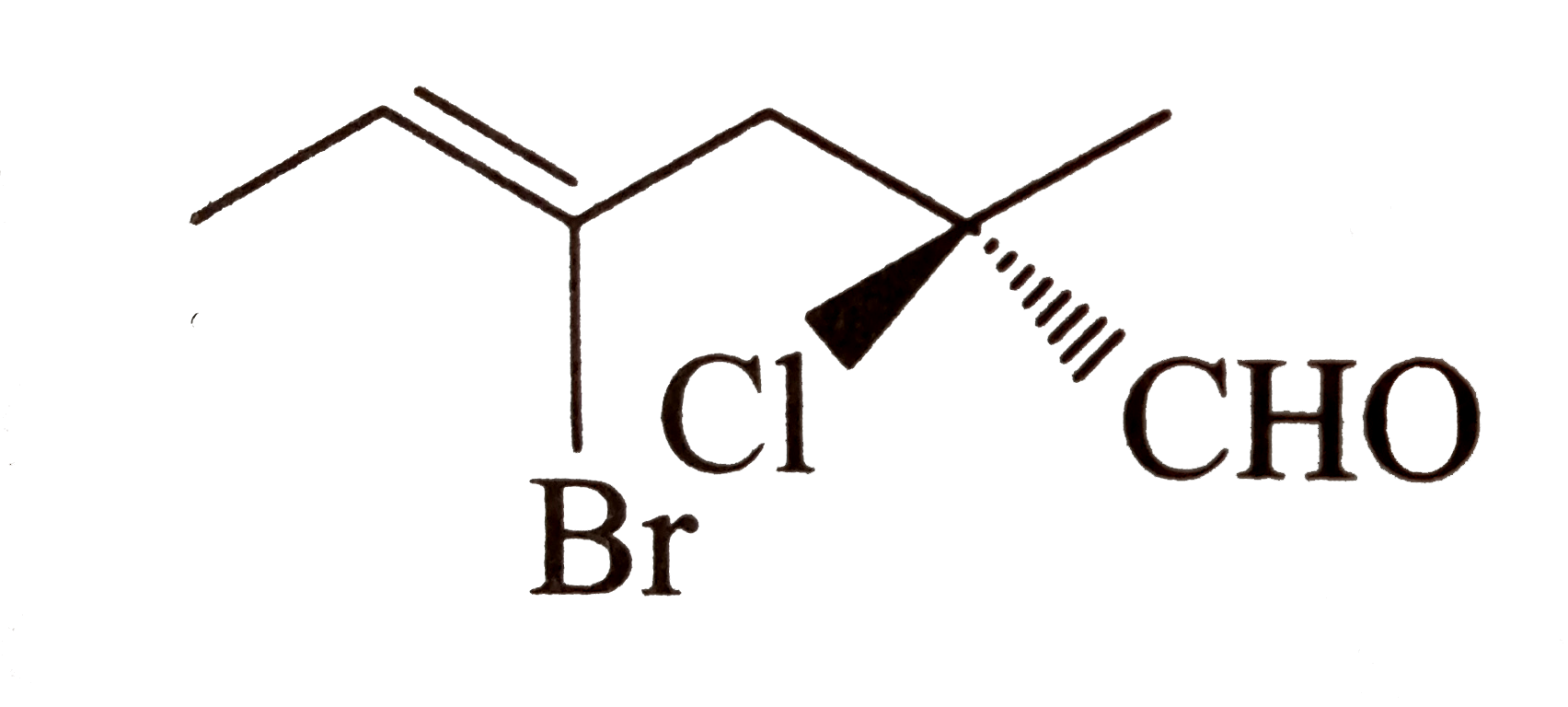 is A(2S,4E)−4−bromom-2-chlor-2-metyhy1hex-4-enalB(2R,4Z)−4−bromo-2-chlor-2-methy1hex-4-enalC(2Z,5R)−3−bromo-5-chlor-5-formy1hex-2-eneD(2R,4E)−4−bromo-2-chloro-2-methy1hex-4-enal
is A(2S,4E)−4−bromom-2-chlor-2-metyhy1hex-4-enalB(2R,4Z)−4−bromo-2-chlor-2-methy1hex-4-enalC(2Z,5R)−3−bromo-5-chlor-5-formy1hex-2-eneD(2R,4E)−4−bromo-2-chloro-2-methy1hex-4-enalThe correct IUPAC name of
 isA1-cyclopropylcyclobutaneB1,1-DicyclobutaneC1-Cyclobutan-1-cyclopropaneDNone of the above
isA1-cyclopropylcyclobutaneB1,1-DicyclobutaneC1-Cyclobutan-1-cyclopropaneDNone of the aboveThe correct IUPAC name is
A(1) CH3−C∣∣O−CH∣CH3−CH32−Bromo-3-chlorobutaneB(2) CH3−CH∣CH3−CH∣CH2CH3CH32,3−DimethylpentaneCCH3C≡CCH(CH3)24−Methylpent-2pneD(4) CH3−CH∣Cl−CH∣Br−CH32,3−Bromo-3-chlorobutane

 is
is