Hexafluorocbaltate (III) ion is an outer orbital complex. The number of unpaired electrons present in it is
A
1
B
5
C
4
D
unpredictable.
Video Solution
Text Solution
The correct Answer is:B
Fe3+ : [Ar]3d5 undergoes sp3d2 -hybridisation. There are five unpaired electrons in the complex.
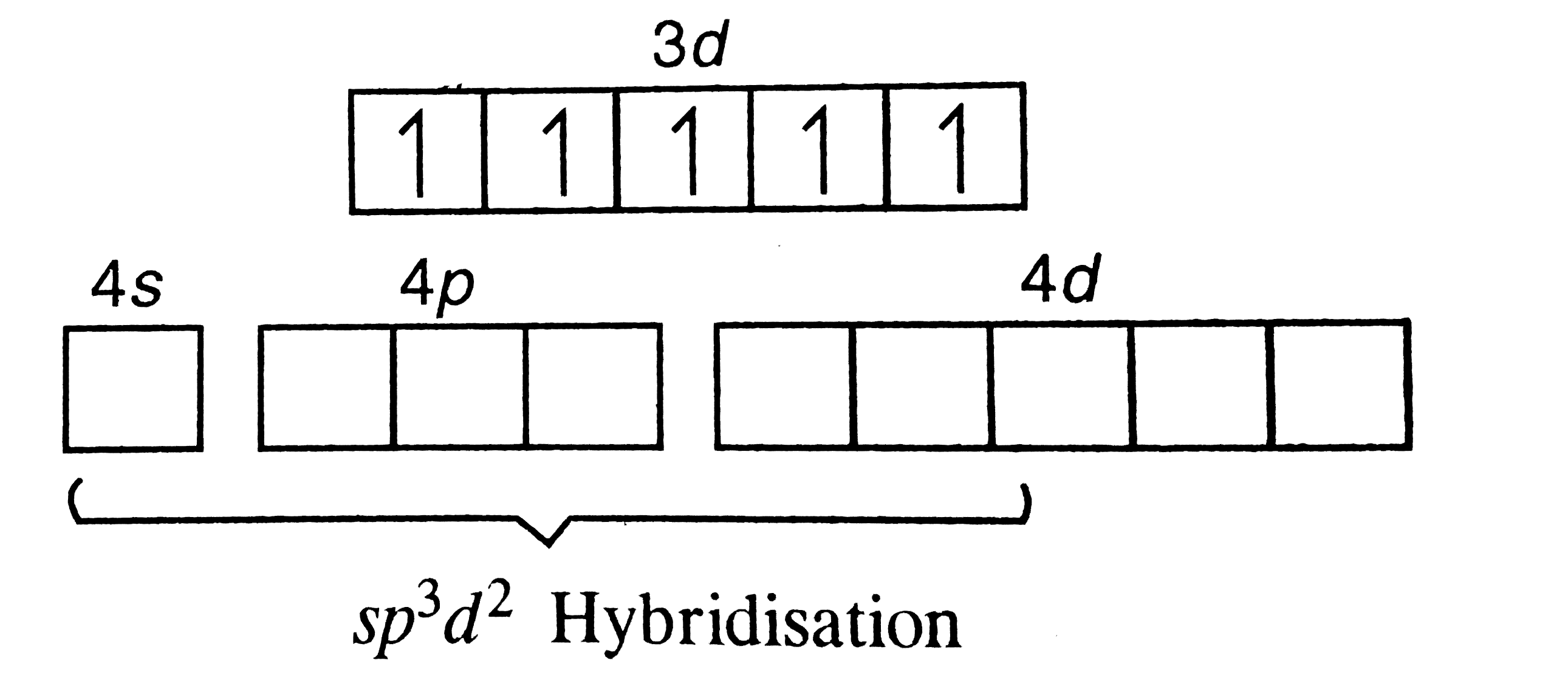
|
Similar Questions
Knowledge Check
Hexafluoroferrate (III)ion is an outer orbital complex. The number of unpaired electrons present in it
A1B4C5D0The number of unpaired electrons present in Am are
A2B3C4D7Outer orbital complex is
A[Zn(NH3)4]2+B[Co(NH3)6]3+C[Cr(NH3)6]3+D[CoF6]3−
The number of unpaired electron(s) in Mg2+ ion is
View SolutionThe number of unpaired electrons in ferrous ion is
View SolutionThe maximum number of unpaired electrons is present in
View SolutionThe number of unpaired electrons in Ce3+ ion is 0
View SolutionThe number of unpaired electrons in Cr3+ ion is
View Solution
