In any ferric salt on adding potassinum ferrocyanide, a prussian blue colour is obtanied, which is mainly due to the formation of
A
K3[Fe(CN)6]
B
Kfe[Fe(CN)6]
C
FeSO4.Fe(CN)6
D
Fe4[Fe(CN)6]3.
Video Solution
Text Solution
The correct Answer is:B
Fe3++K4[Fe(CN)6]Potassium ferrocyanide→KFe[Fe(CN)6]3Prussian blue+3K+
|
Topper's Solved these Questions
- CO-ORDINATION COMPOUNDS Book:DINESH PUBLICATIONChapter:CO-ORDINATION COMPOUNDS Exercise:SELECTED STRAIGHT OBJECTIVE TYPE MCQS
Explore 84 Videos
- CO-ORDINATION COMPOUNDS Book:DINESH PUBLICATIONChapter:CO-ORDINATION COMPOUNDS Exercise:COMPREHENNSION TYPE MCQS
Explore 5 Videos
- CO-ORDINATION COMPOUNDS Book:DINESH PUBLICATIONChapter:CO-ORDINATION COMPOUNDS Exercise:Brain storming multiple choice question
Explore 12 Videos
- CHEMISTRY IN EVERY DAY LIFEBook:DINESH PUBLICATIONChapter:CHEMISTRY IN EVERY DAY LIFEExercise:Unit test-9
Explore 50 Videos
- CYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINESBook:DINESH PUBLICATIONChapter:CYANIDES, ISOCYANIDES, NITROCOMPOUNDS AND AMINESExercise:Unit test - 8
Explore 20 Videos
Similar Questions
Knowledge Check
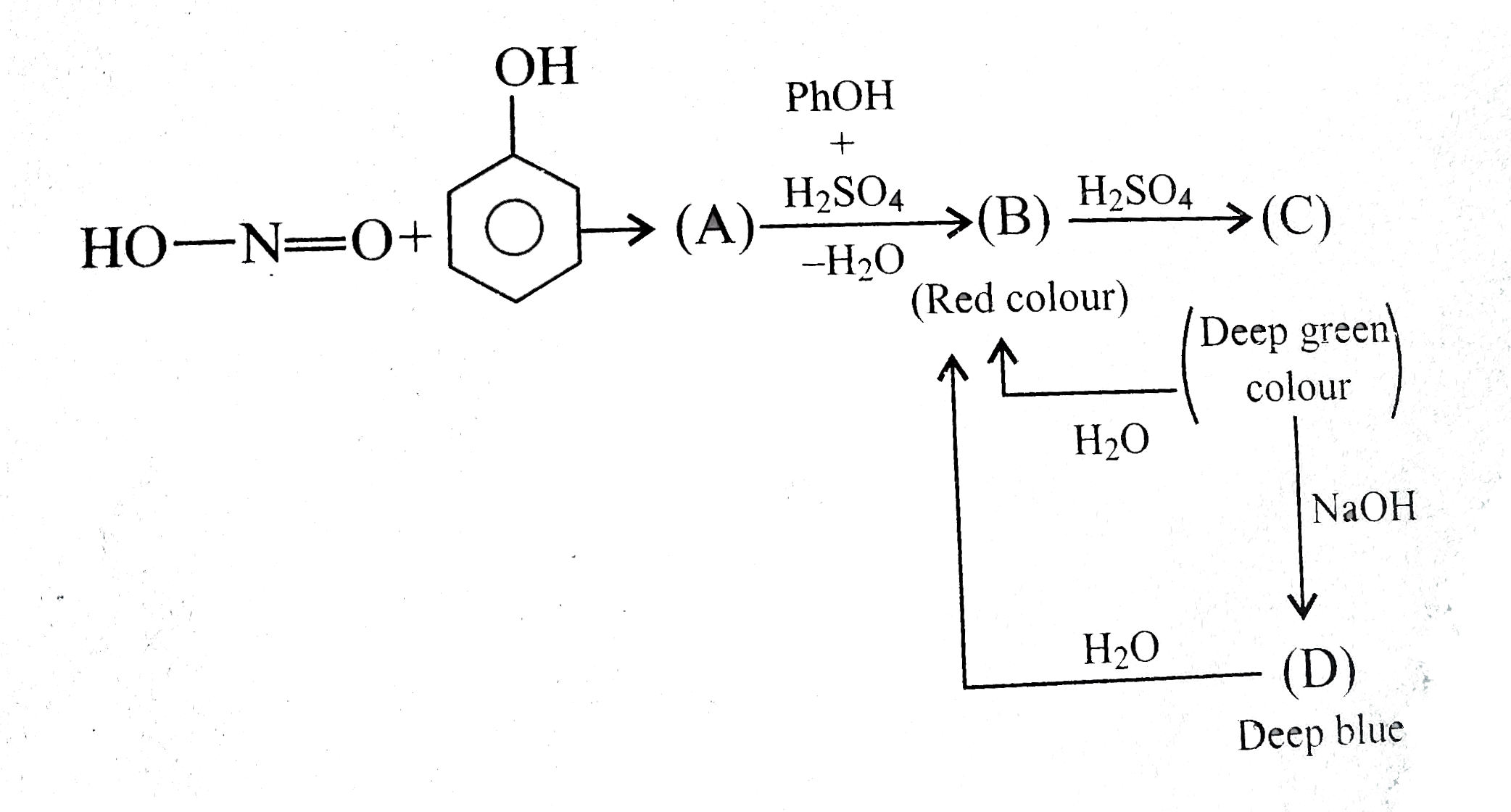
The deep blue colour of compound (D) is due to the formation of:Aa.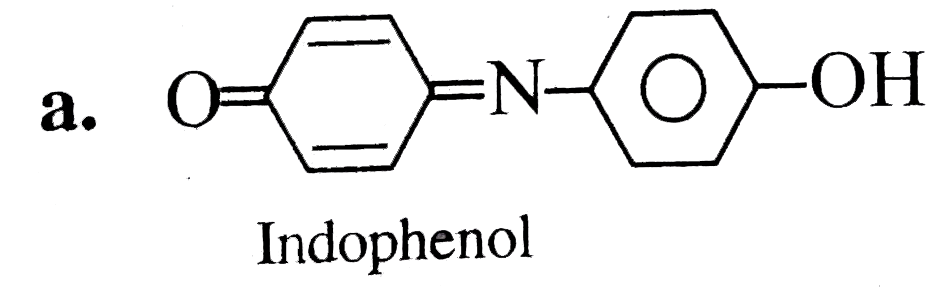 Bb.
Bb. Cc.
Cc. Dd.Both (a) and (b).
Dd.Both (a) and (b).Colour of the bead in borax bead testis mainly due to the formation of
Ametal oxidesBboron oxideCmetal metaboratesDelemental boron.Ferric ion forms a prussian blue coloured solution with K4[Fe(CN)6] due to the formation of -
AK3Fe(CN)6BFe4[Fe(CN)6]3CFe(OH)3DKFe[Fe(CN)6]
In the Lassaigne's test for nitrogen in an organic compound, the Prussian blue colours is obtained due to the formation of:
View SolutionThe Prussian blue colour obtained in the Lassaigne' s test for nitrogen is due to the formation of
View SolutionThe prussian blue colour obtained in the Lassaigne's test for nitrogen is due to formation of :
View SolutionIn the Lassaigne's test for nitrogen in an organic compound, the Pressian blue colour is obtained due to the formation of :
View SolutionIn the borax test of Co2+ , the blue colour of bead is due to the formation of
View Solution
