In thistle funnel experiment, if sugar is added to beaker after the stoppage f osmosis, then
Level of solution in beaker lowers
Level of solution in thishtle funnel rises up
Level of solution remins unaffected in beaker
Level of solution in thistle funnel lowers.
The correct Answer is:D
Step by step video, text & image solution for In thistle funnel experiment, if sugar is added to beaker after the stoppage f osmosis, then by Biology experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- TRANSPORT IN PLANTSBook:DINESH PUBLICATIONChapter:TRANSPORT IN PLANTSExercise:CHECK YOUR GRASP
Explore 22 Videos
- TRANSPORT IN PLANTSBook:DINESH PUBLICATIONChapter:TRANSPORT IN PLANTSExercise:CHECK YOUR GRASP
Explore 22 Videos
- THE LIVING WORLDBook:DINESH PUBLICATIONChapter:THE LIVING WORLDExercise:Check your grasp
Explore 12 Videos
- VERTEBRATA-AGNATHA AND PISCESBook:DINESH PUBLICATIONChapter:VERTEBRATA-AGNATHA AND PISCESExercise:Check Your Grasp
Explore 9 Videos
Similar Questions
In an experiment to determine the boiling point of water state the reason for the following recautions:
(i) The bulb of the thermometer should not touch the sides of the beaker.
(ii) while boiling water pumice stone should be added.
View SolutionThe reaction An+CuSO4→ZnSO4 goes completely to the right. In an experiment, 10.0 g of Zn was added to 200 " mL of " CuSO4 solution. After all the Cu was precipitated, it was found that not all the Zn had dissolved. After filtration, the total weight of the solid at the end of the reaction was 9.810 g. Calculate.
(a). The weight of copper deposited and,
(b). Molarity of CuSO4 in the original solution.
View Solution
Knowledge Check
In thistle funnel experiment, during osmosis the level of the solution in the funnel
AIncreasesBDecreasesCRemains sameDFirst increases then decreasesIn thistle funnel experiment, entry of water into thistle funnel stops after some time autumatically due to
ADiffusion of sugar out of thistle funnelBExternal and internal solutions become isotonicCDevelopment of hyrostatic pressure in the thistle funnelDDevelopment of gydrostatic pressure in the beaker.In the given experiment, some sea water was heated in a beaker. After some time, it was observed that the number of water droplets formed on the aluminium sheet become less and less. Why was this so?
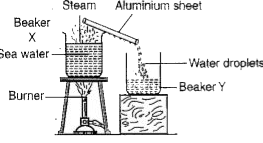
(i) The aluminium sheet had become hotter
(ii) The rate of condensation had decreased
(iii) Beaker X had become hotterA(i) and (iii) onlyB(i) and (ii) onlyC(ii) and (iii) onlyD(i),(ii) and (iii)
During an experiment phenolphthalein is added to an acid solution. After that a basic solution is added dropwise until the complete neutralisation of acid occurs. If one more drop of the basic solution added, then the colour of the acid containing solution would turn to
View SolutionDuring an experiment phenolphthalein is added to an acid solution. After that a basic solution is added dropwise until the complete neutralisation of acid occurs. If one more drop of the basic solution added, then the colour of the acid containing solution would turn to
View SolutionAn unknown liquid collected from a sample of peas, is added to a beaker of water and is vigorously shaken. After few minutes, water and the unknown liquid made two separate layers. To which class of biomolecules, does the unknown liquid most likely belongs ?
View SolutionTwo equally charged small metal balls placed at a fixed distance experience a force F. A similar unchanged metal ball after touching one of them is placed at the middle point between the two balls. The force experienced by this ball is
View SolutionWhen 100 mL of 1.0 M HCl was mixed 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase 5.7∘C was measured for the beaker and its contents (Expt. 1). Because the enthalpy of neutralization of a strong acid with a strong base is a constant (−57.0kJmol−1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt.2) 100 mL of 2.0 M acetic acid (Ka=2.0×10−5) was mixed with 100 mL of 1.0 M NaOH (under indentical conditions to Expt. 1) where a temperature rise of 5.6∘C was measured
(Consider heat capacity of all solutions as 4.2Jg−1K−1 and density of al solutions as 1.0 gmL−1)
The pH of the solution after Expt. 2 is
View Solution
