Give the chemical composition of the following :
(a) Anhydrone (b) Fluid magnesia (c) Dead burnt plaster (d) Sorel Cement (e) Epsom salt (f) Magnesia mixture
(a) Anhydrane : It is magnesium perchlorate with the formula Mg(CIO4)2.
(b) Fluid magnesia : A 12% aqueous solution of Mg(HCO3)2 is called fluid magnesia.
(c ) Dead burnt plaster : It is anhydrous CaSO4
(d) Sorel Cement : It is a hard mass generally used in dental filling. It is a paste of finely divided magnesium oxidation magnesium chloride along with fillers like soap stone and wood fluor. The chemical formula is MgCI2.5MgOO.xH2O
(e) Epson salt : It is hydrated magnesium sulphate wit formula MgSO4.7H2O.
(f) Magnesia mixture : It is a mixture of MgCI2, NH4CI used small amount of NH3. It is used for the detetion of phosphate (PO3−4) and aresenate (AsO3−3) in qualitative analysis.
Similar Questions
Give the chemical composition of the following :
(a) Anhydrone (b) Fluid magnesia (c) Dead burnt plaster (d) Sorel Cement (e) Epsom salt (f) Magnesia mixture
View SolutionGive the decreasing order of acidic character of the following :
(a)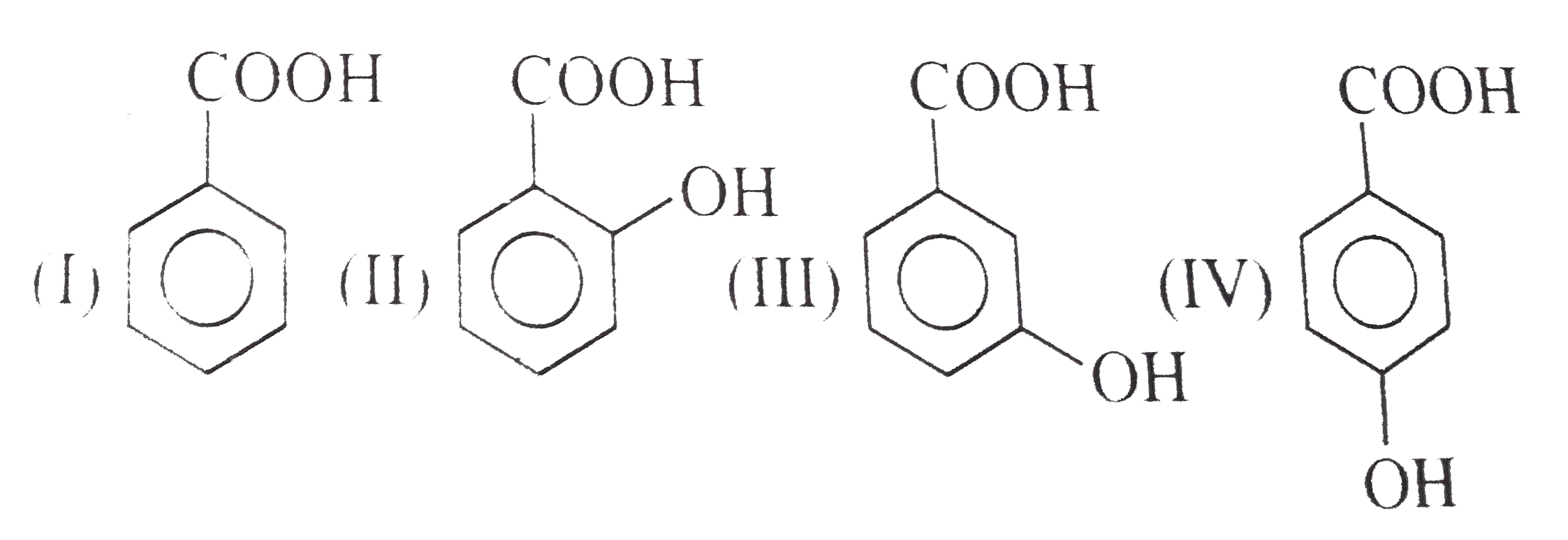
(b)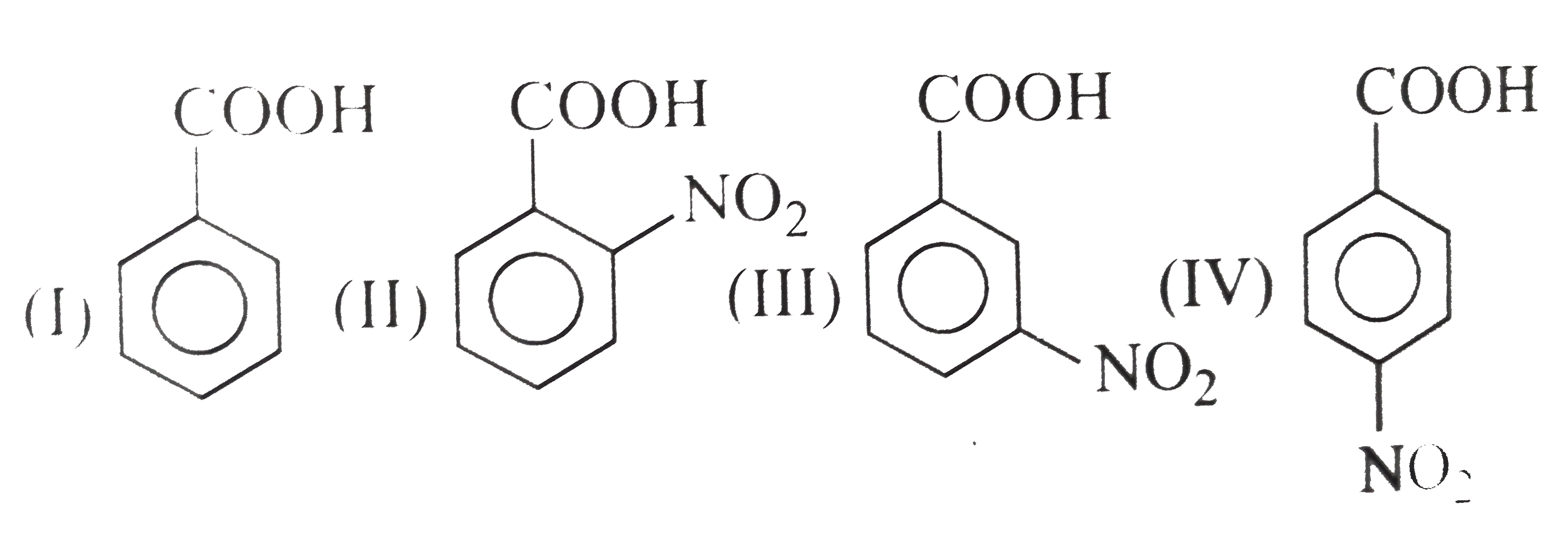
( c)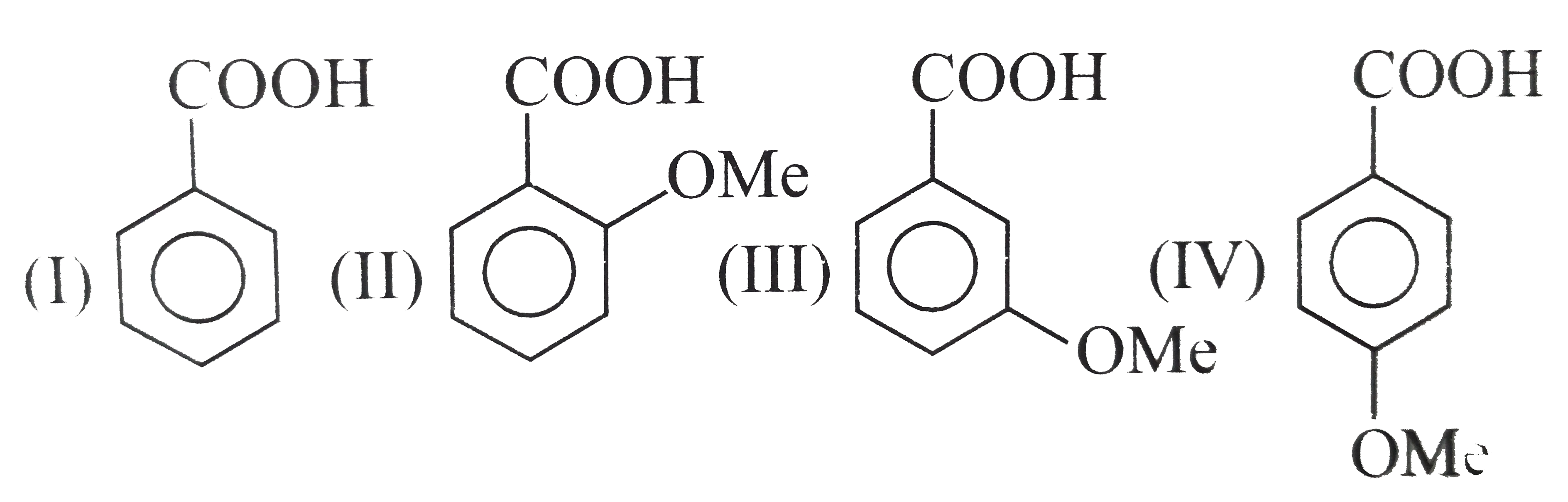
(d)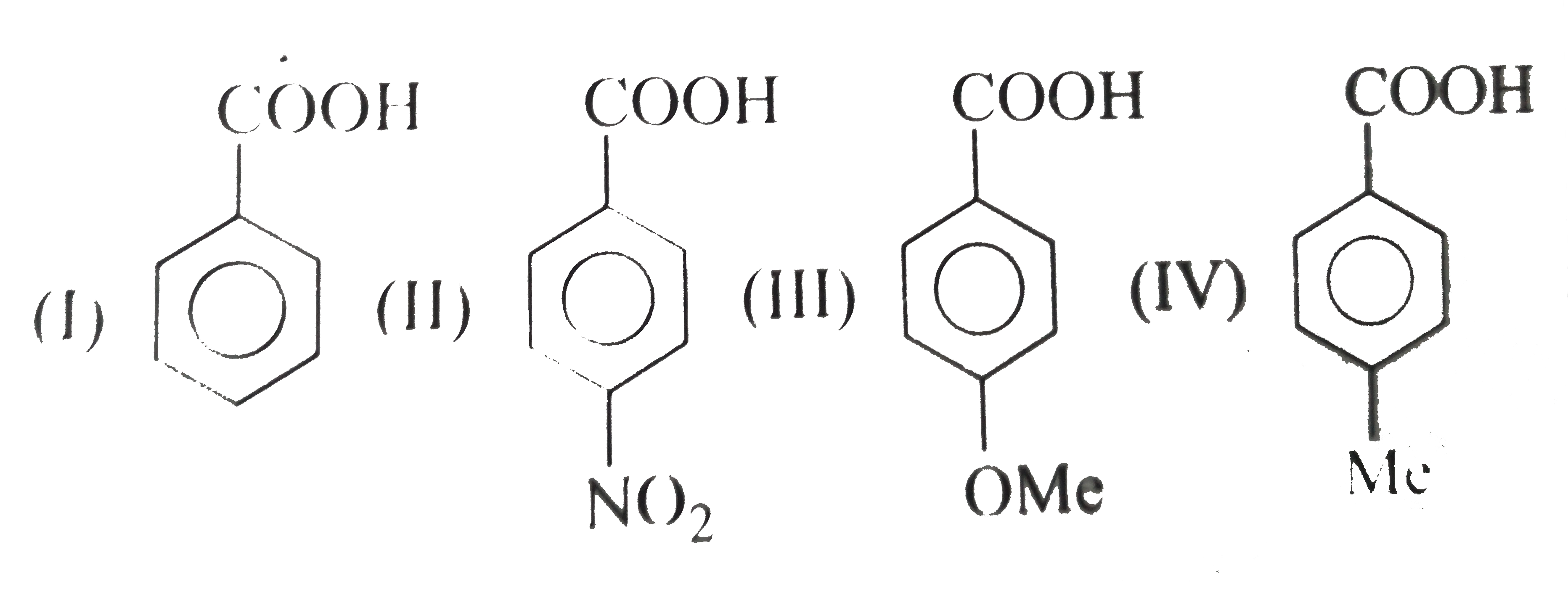
( e)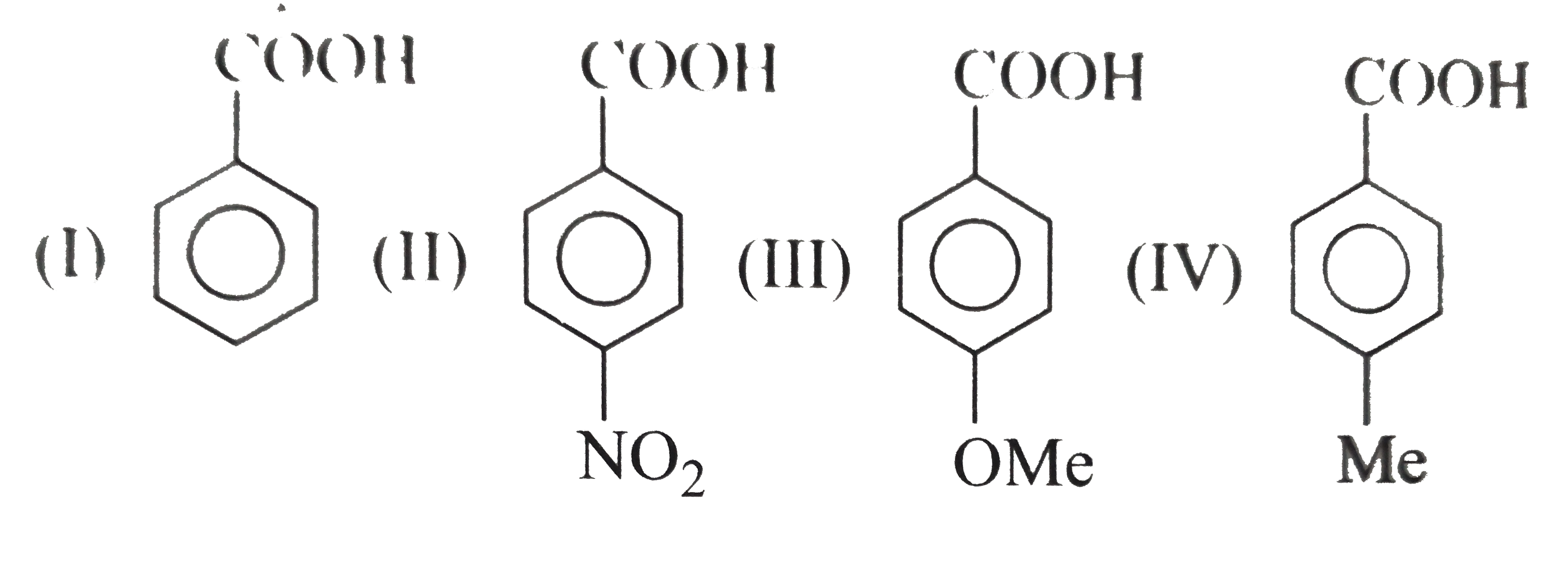
(f)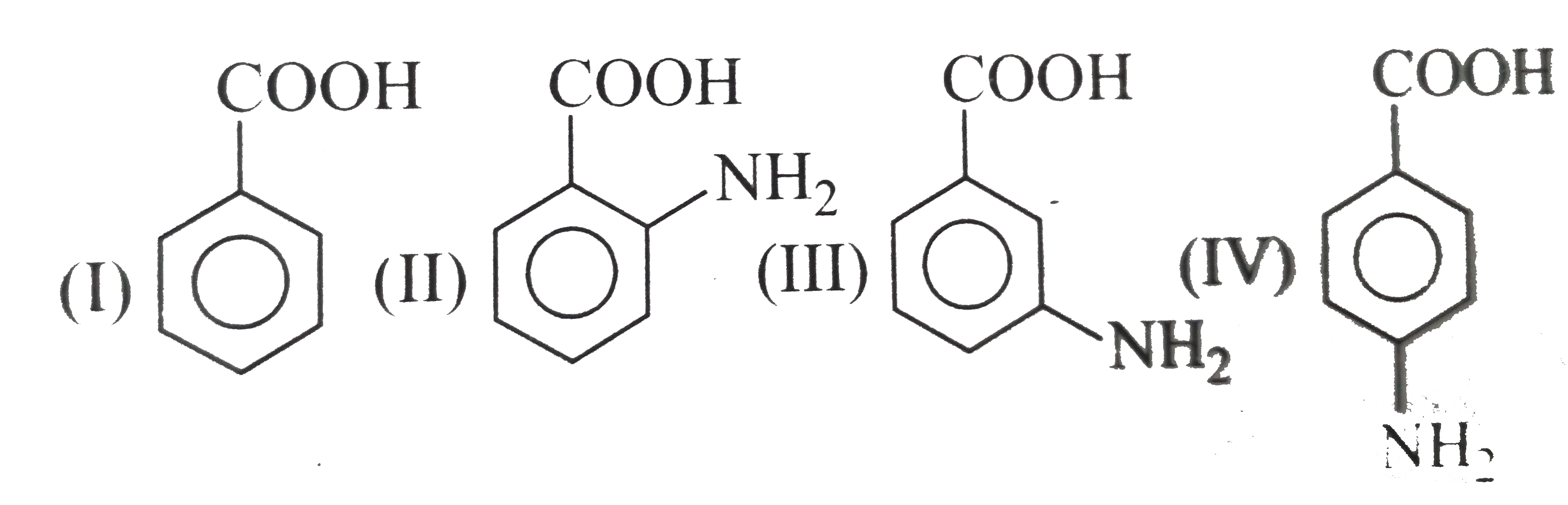 .
.
View SolutionGive the majore and minor products of the following reactions.
a.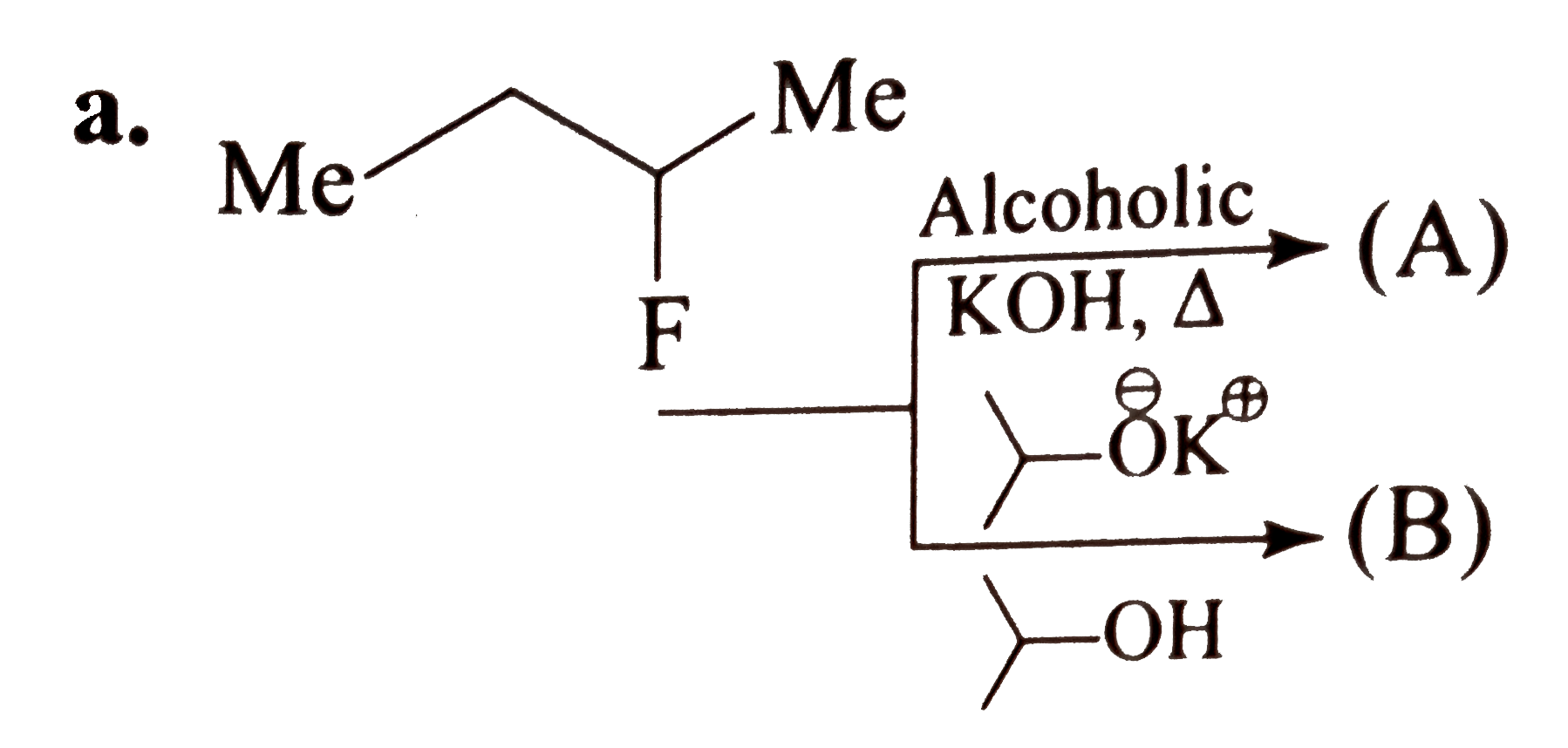
b.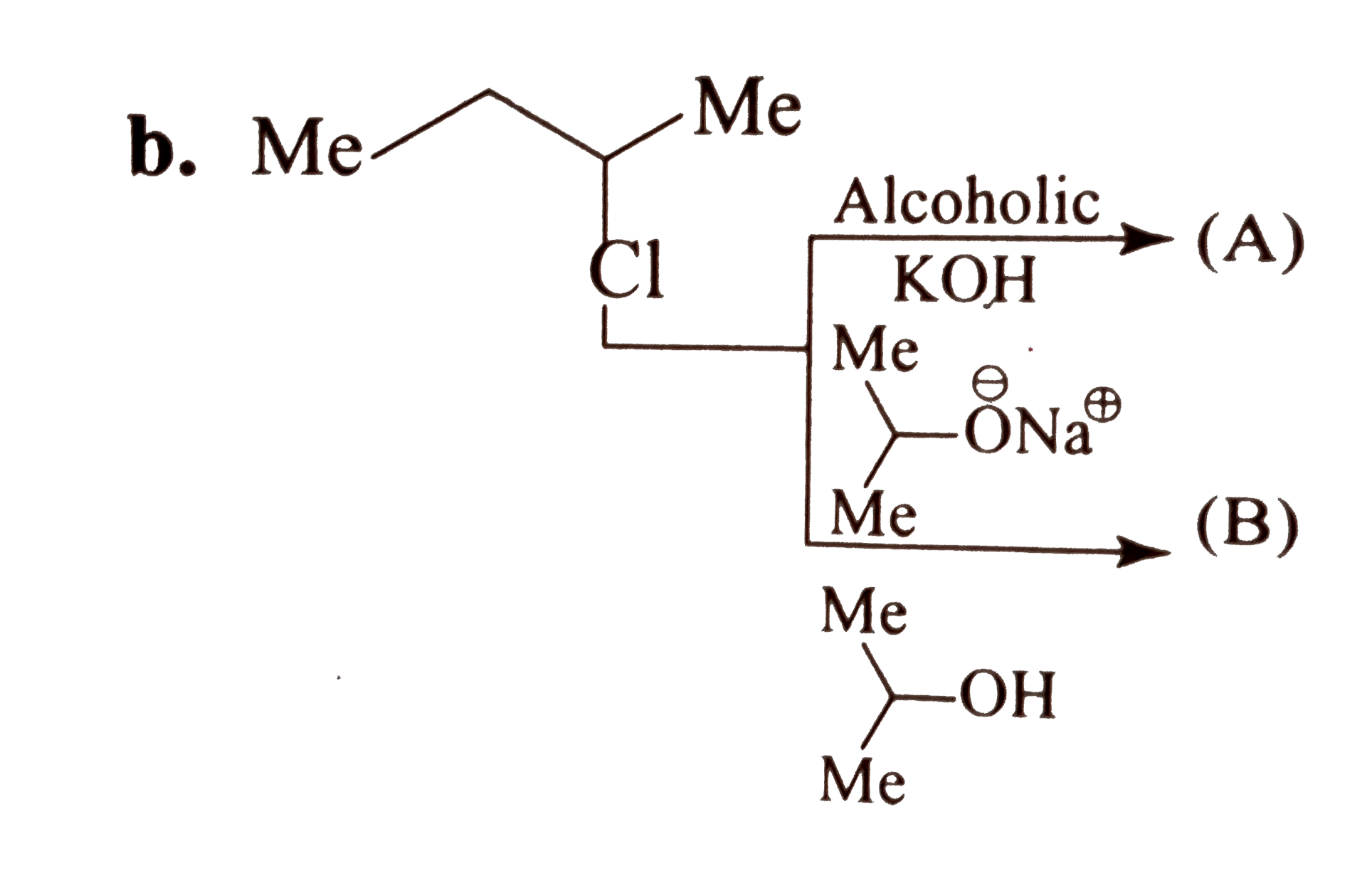
c.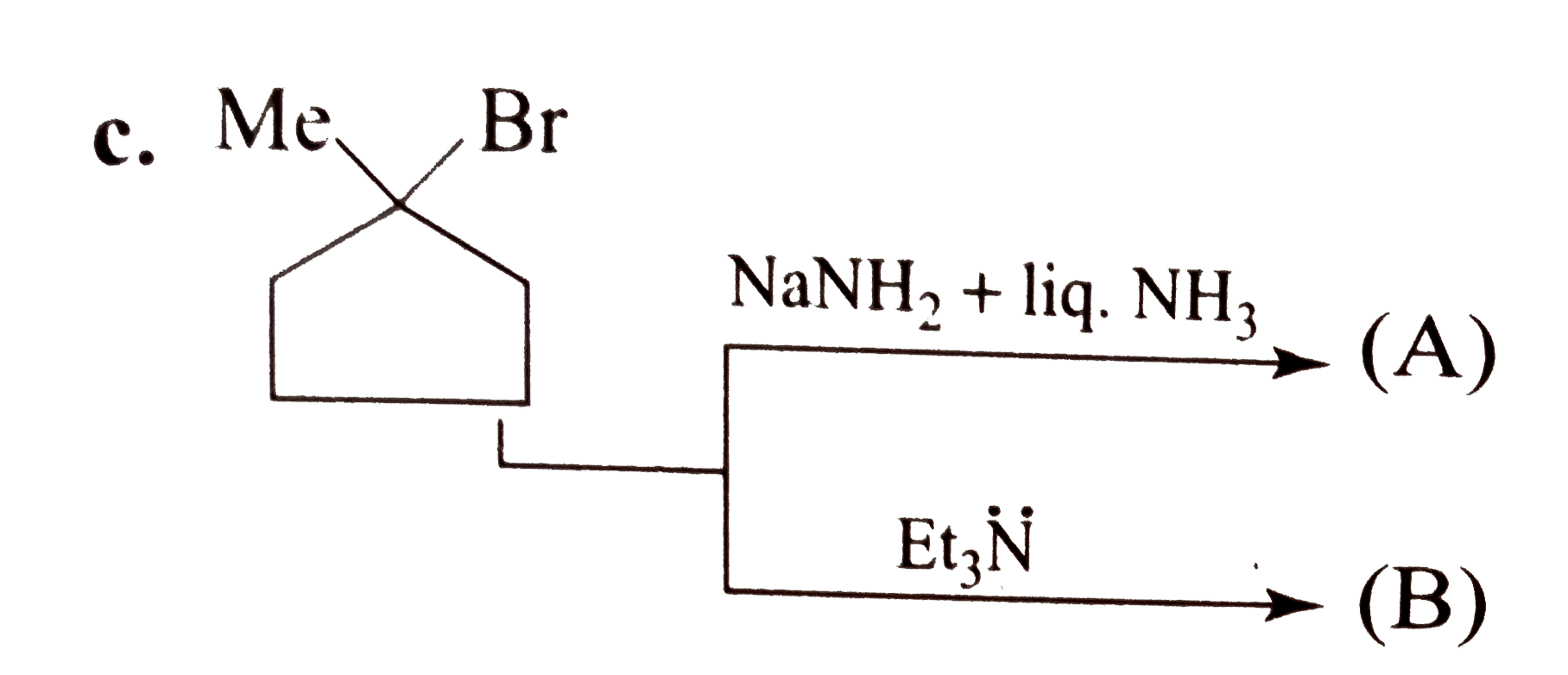
d.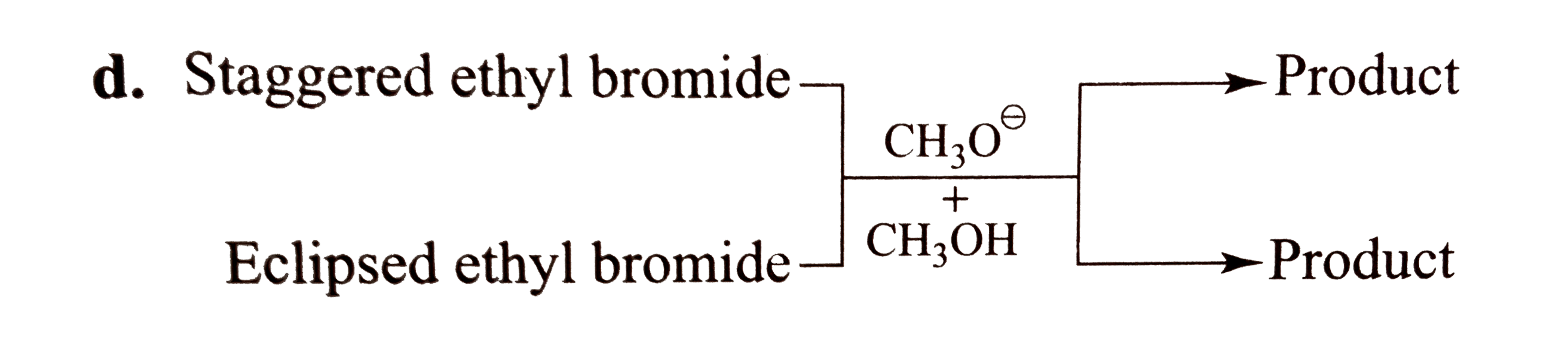
e.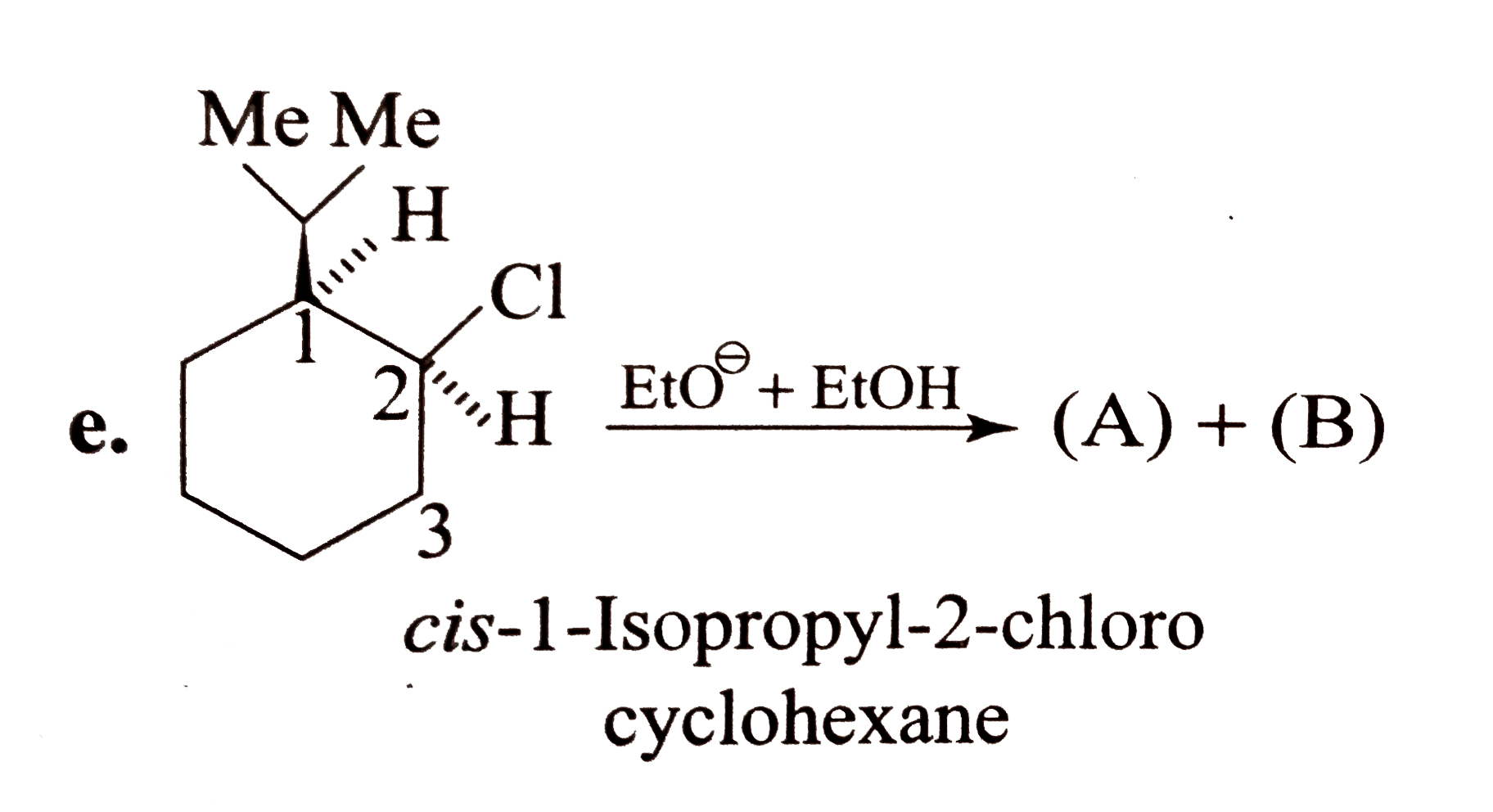
f.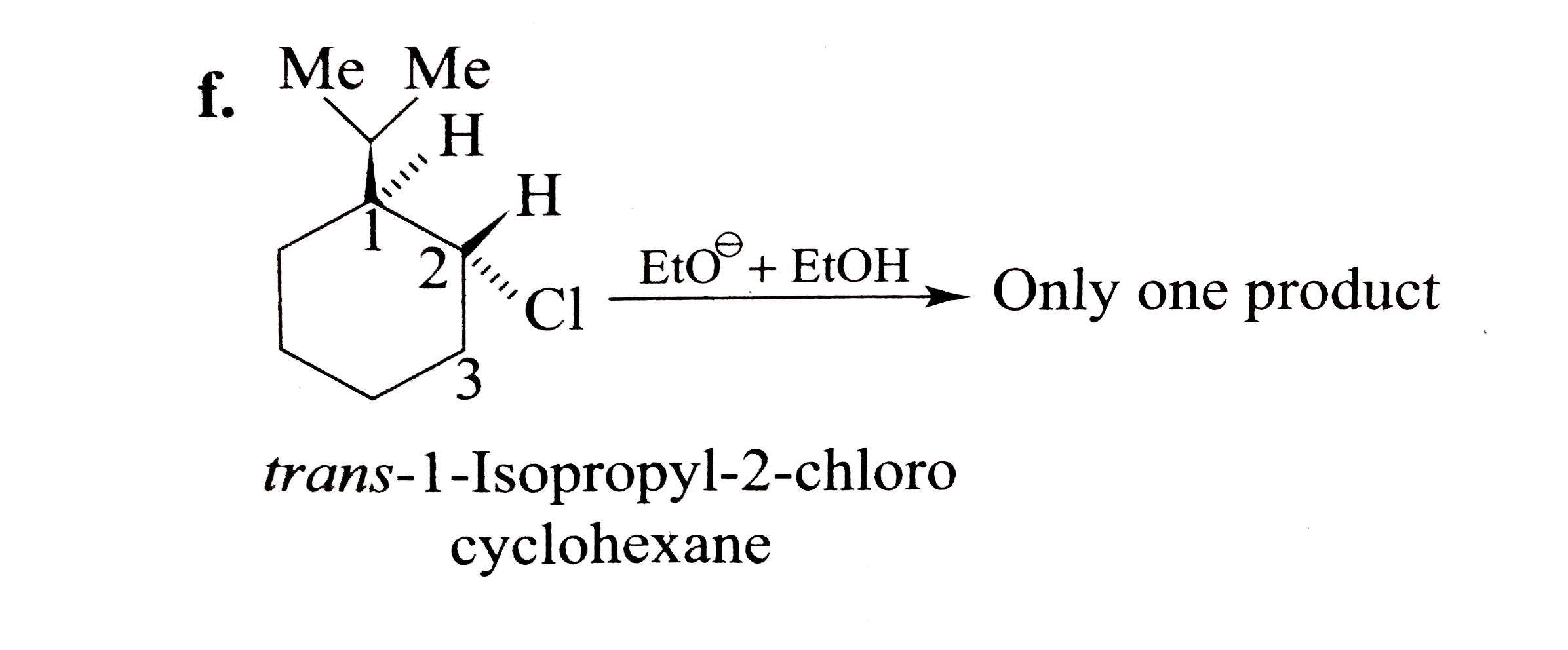
View SolutionFind the values of A,B,C,D,E,F,G in the following
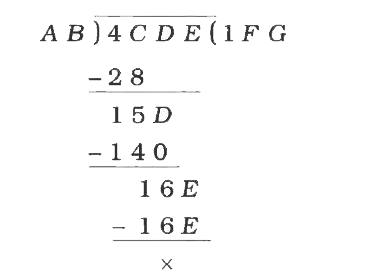
View SolutionIdentify the composite numbers from the following. (a)1(b)2(c)40+13(d)6027(e)331(f)79
View SolutionGive one example for each of the following types
(a) Migratory animal (b) Camouflaged animal
(c)Predator animal (d) Biological control agent
(e)Phytophagous animal (f) Chemical defense agent
View SolutionGive the chemical formula, for each of the following acids :
(a) Nitric acid (b) Hydrogen bromide
(c) Carbonic acid (d) Hypochlorous acid
(e) Iodic acid (f) Sulphurous acid
View SolutionComplete the following reactions:
a.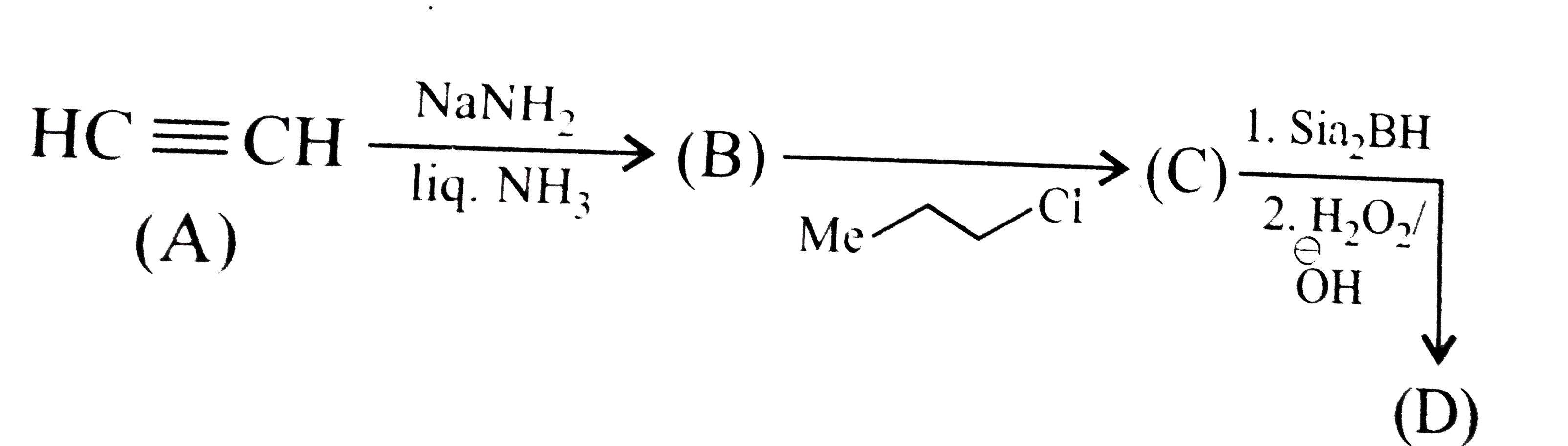
b.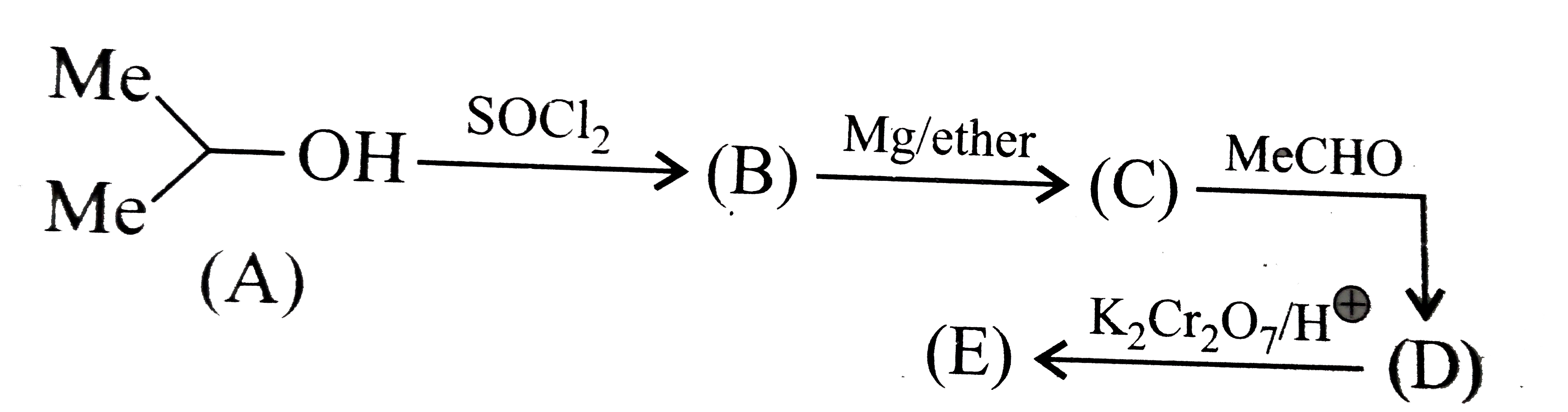
c.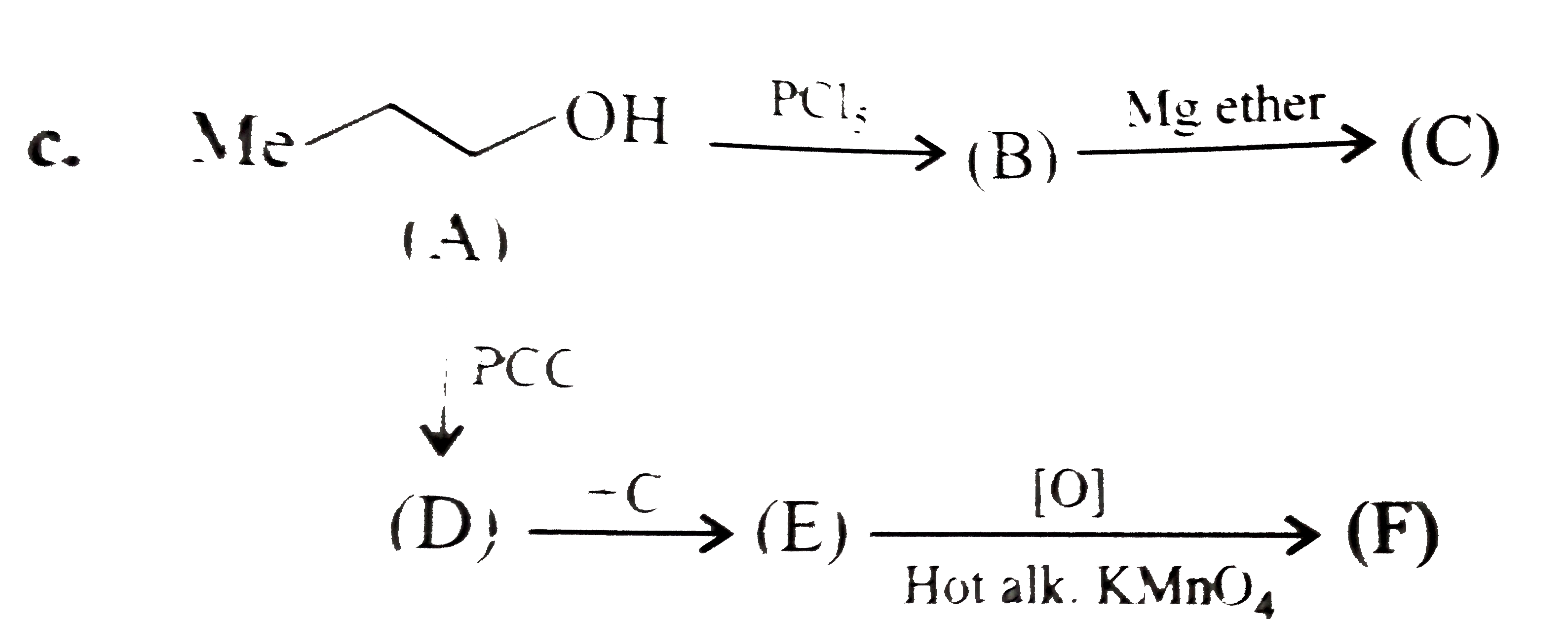
d.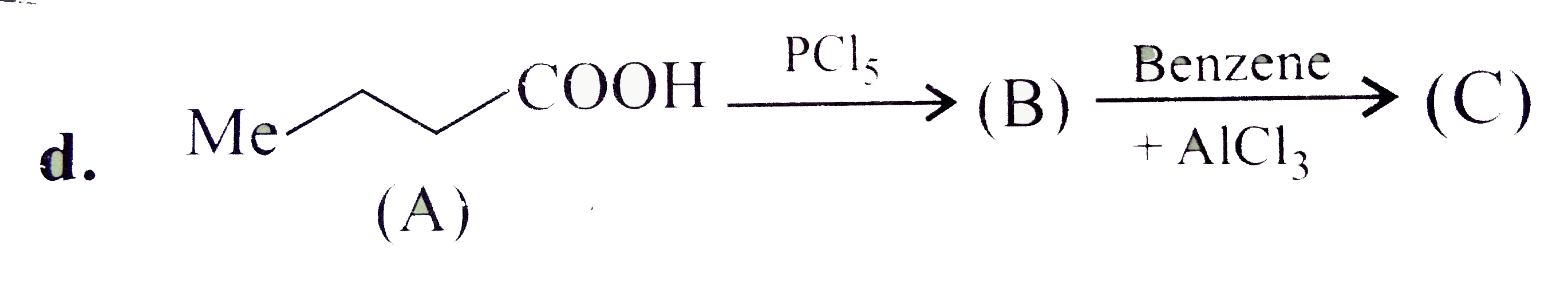
e.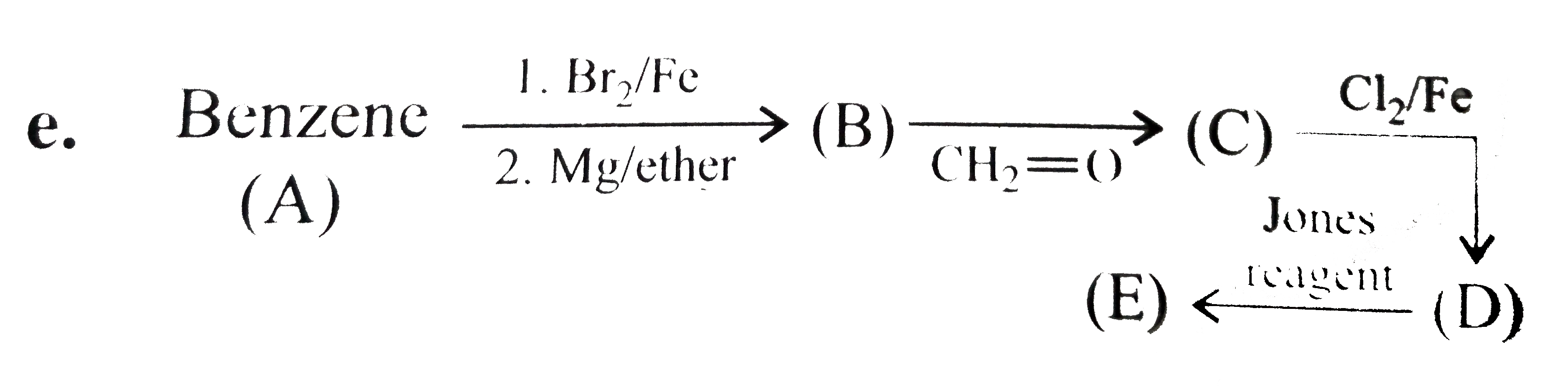
f.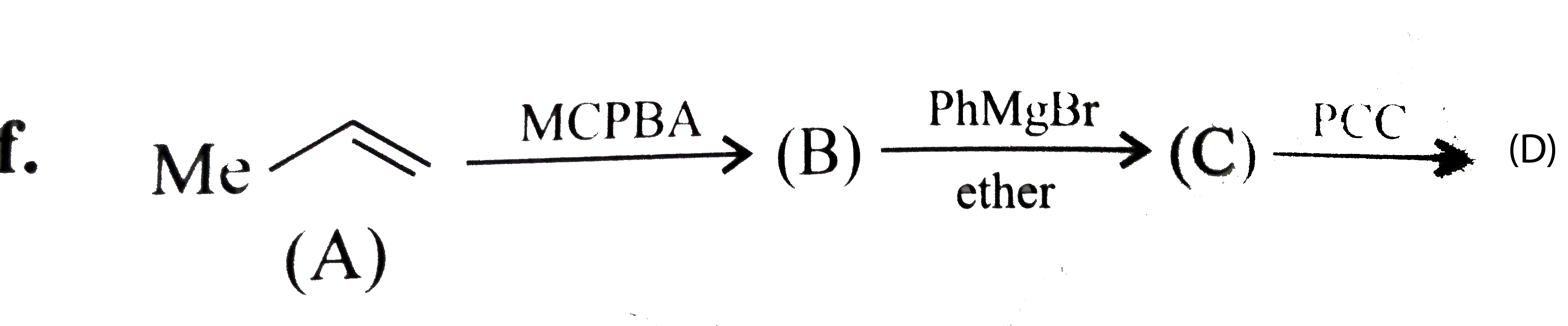
g.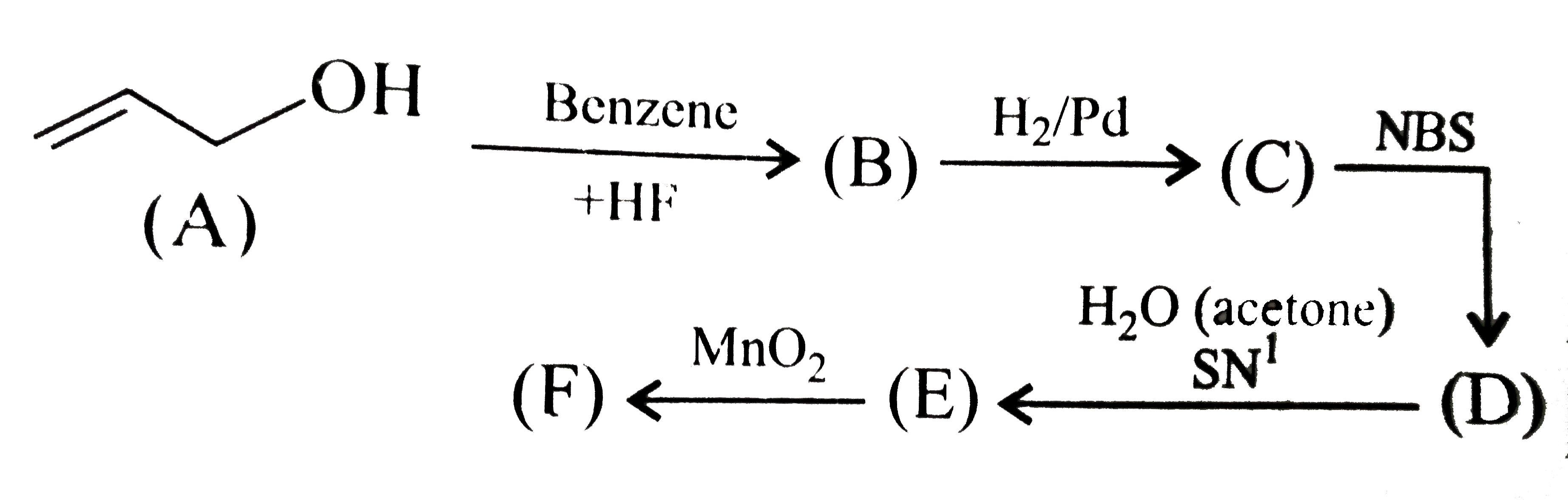
View SolutionComplete the following reactions:
a.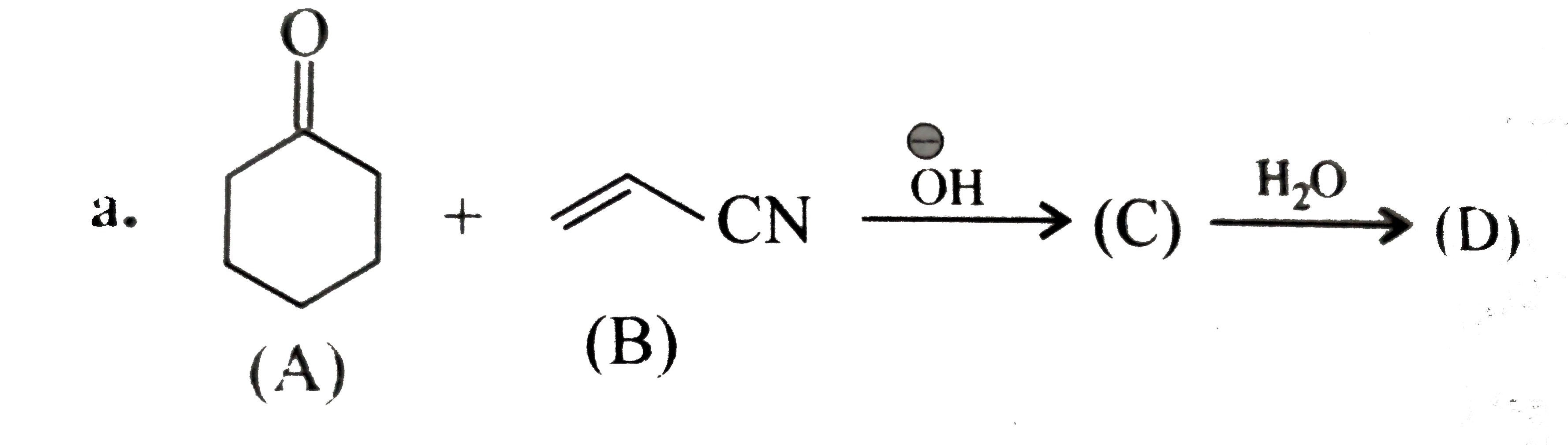
b.
c.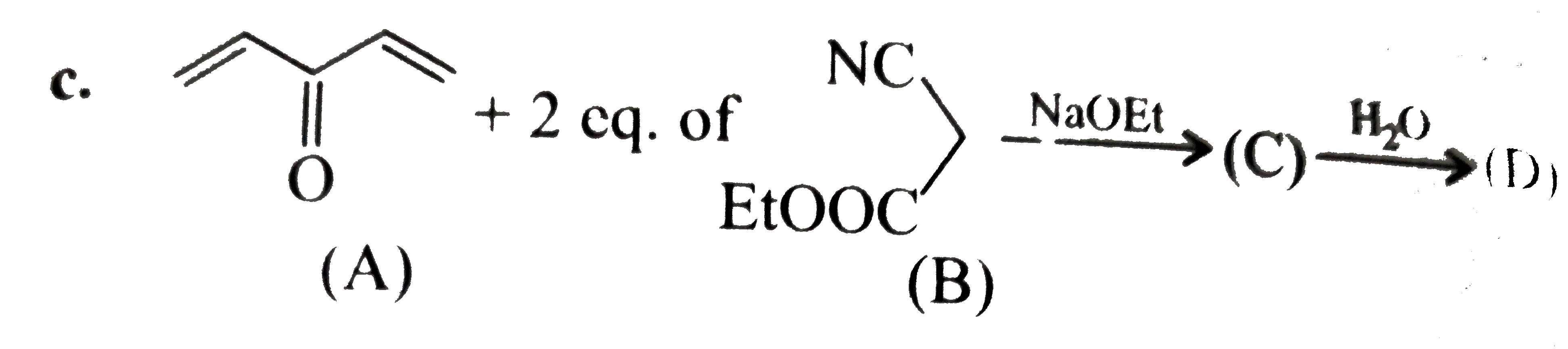
d.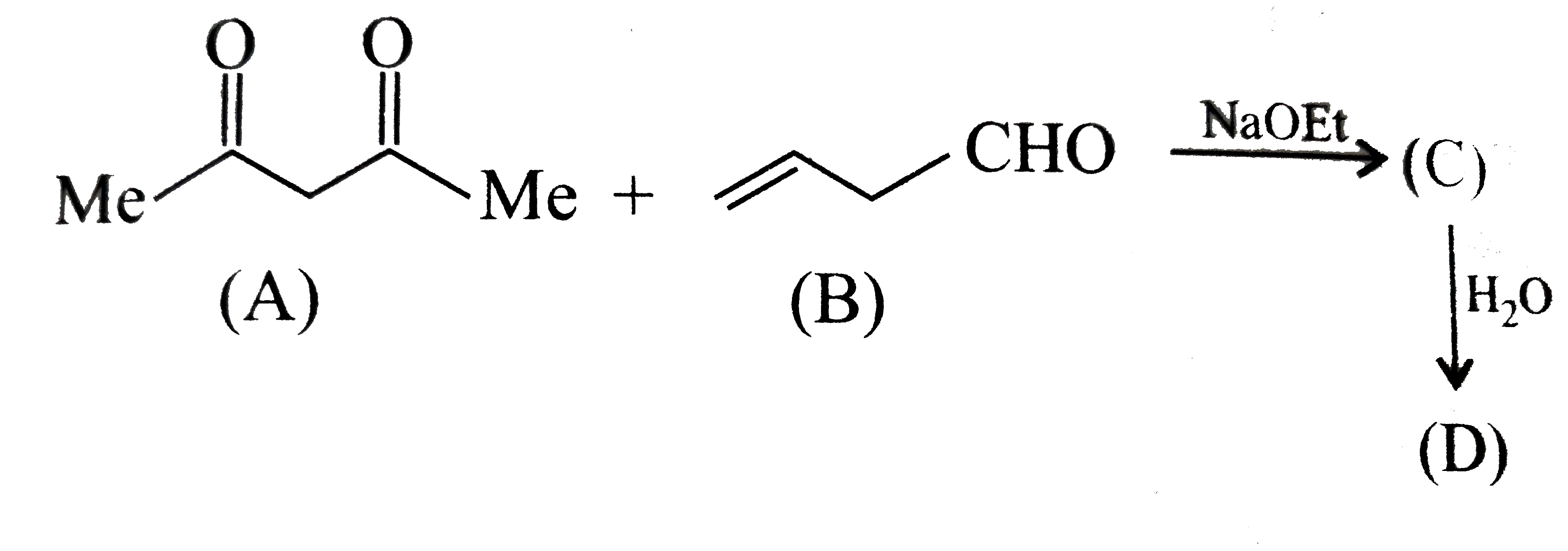
e.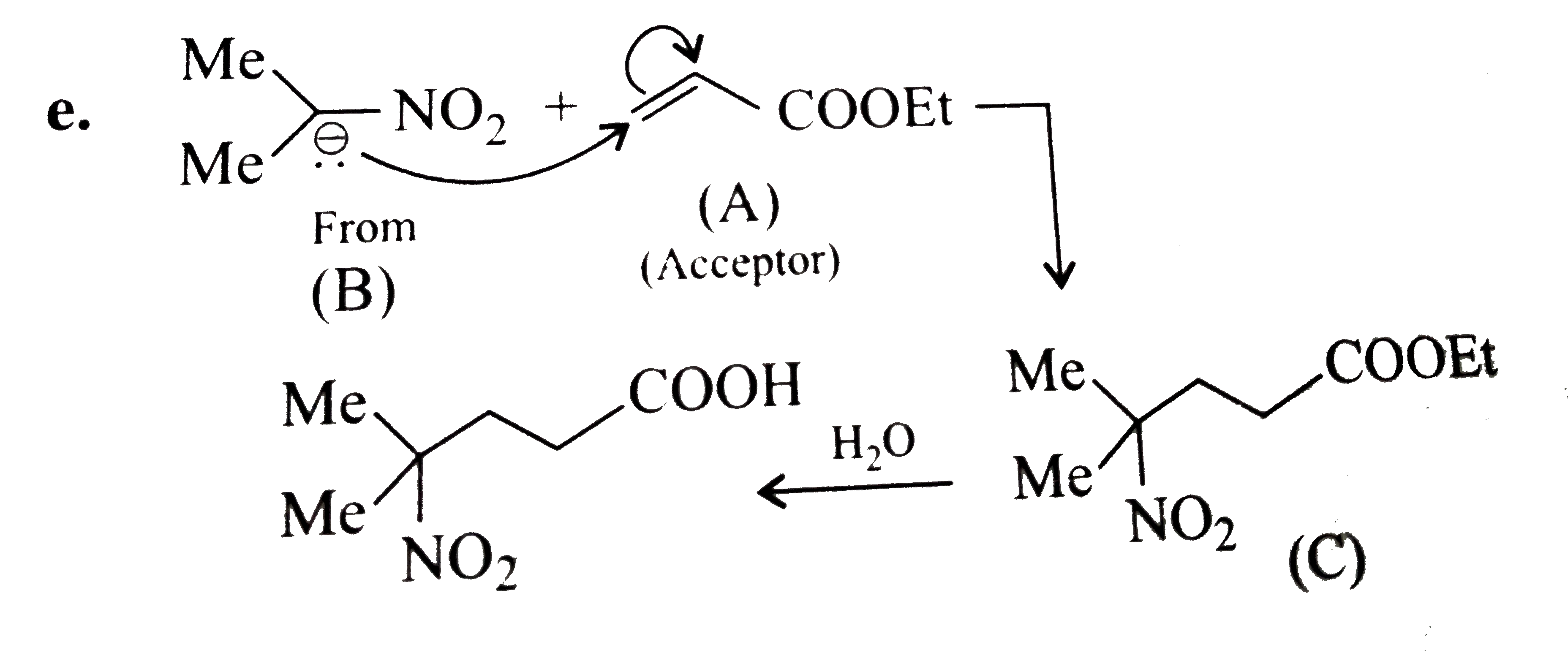
f.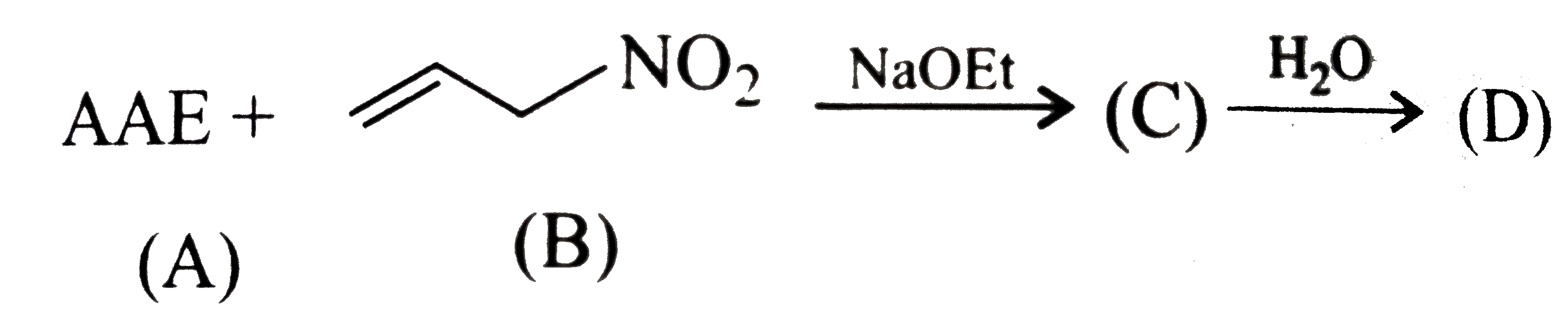
g.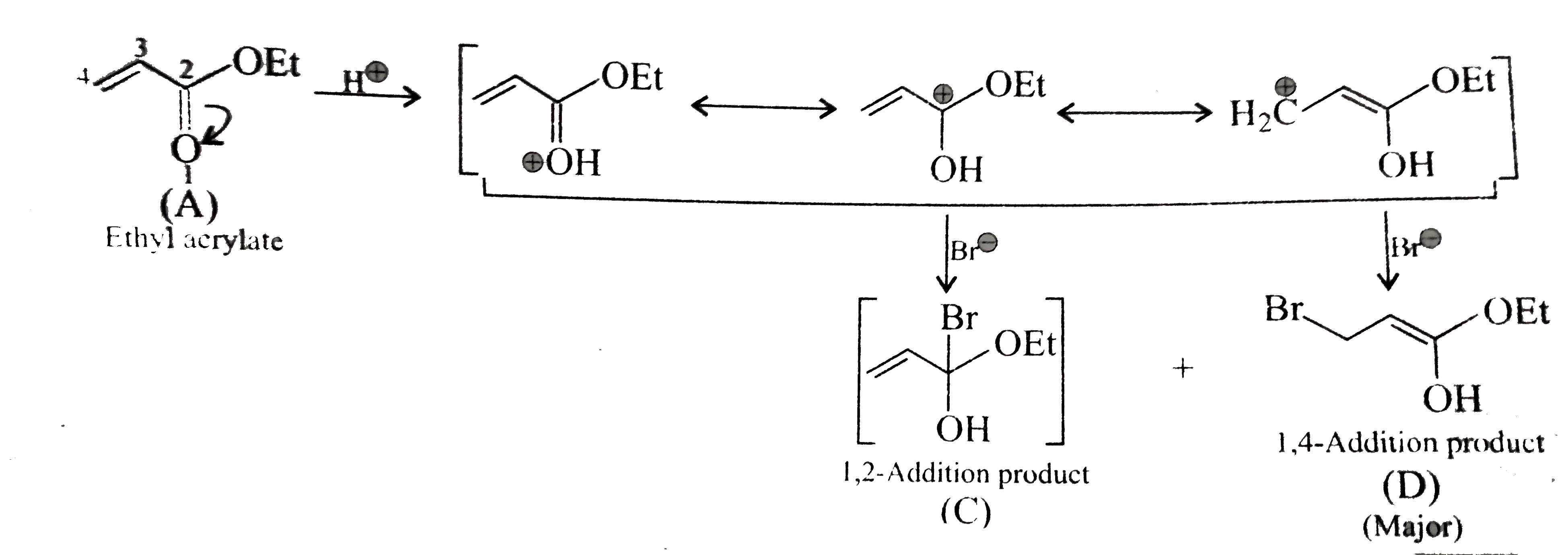
View SolutionDistinguish between the following:
a.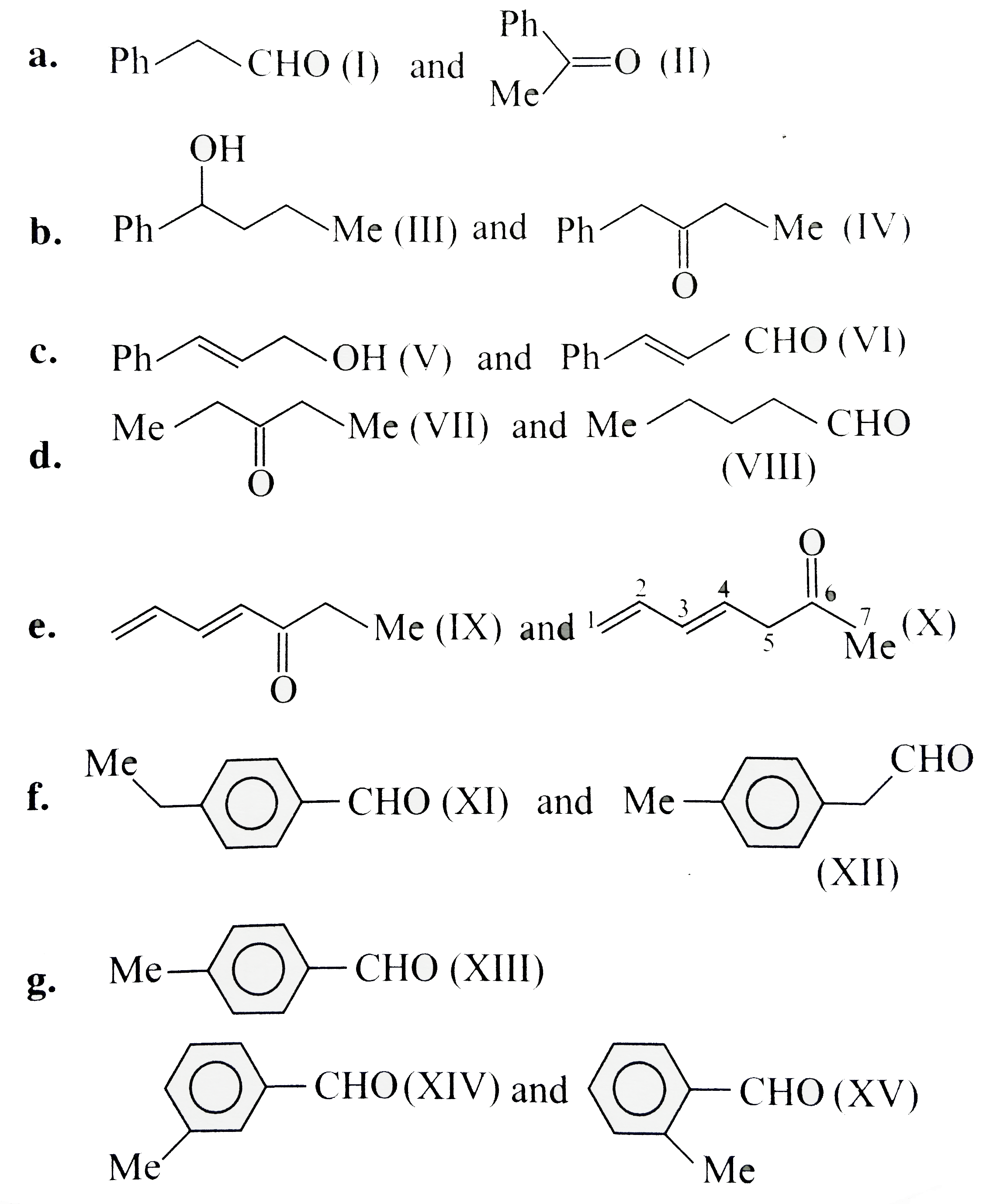
b.
c.
d.
e.
f.
g.
View Solution

