CaSO4.2H2OHeat−−→
Text Solution
CaSO4.2H2OHeat−−→CaSO41/2H2O+3/2H2O
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
The formula of gypsum and plaster of paris are CaSO4.XH2OandCaSO4.Y2H2O respectively. Find the value of x+y.
AGypsumBAnhydriteCAlabasterDPlaster of parisY200∘C←−−−CaSO4.2H2O120∘C−−−→X.
X and Y are respectively:-APlaster of paris, dead burnt plasterBdead burnt plaster, plaster of parisCCaO and plaster of parisDPlaster of paris, mixture of gasesMatch the chemical conversions in List with the appropriate reagent in List II and select the correct answer using the code given below the lists:
List I
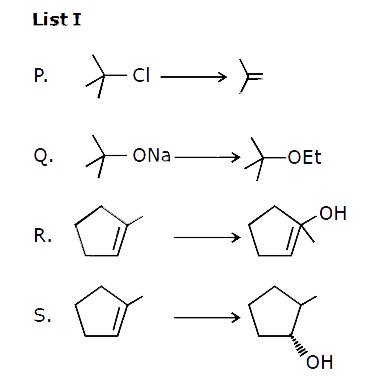
List II
1. (i)Hg(OAc)2,(ii)NaBH2
2. (i) alcKOH
3. Et-Br
4. (i)BH3,(ii)H2O2/NaOHAPQRS1342BPQRS4123CPQRS3124DPQRS4213
Complete the following reactions :
(a) (NH4)2Cr2O7Heat
(b) I−+O3+H2O→
View SolutionCaSO4⋅2H2Oheat−−→373K
View SolutionC overset("प्रबल गर्म करने पर") to B overset(200^(@)C) to CaSO_(4).2H_(2)O overset(120^(@) C)underset("ऊष्मा")to A
उपरोक्त अभिक्रिया में A, B और C क्रमश: हैं:
View SolutionConsider the following sequence of reactions:
Ketone A1.C2H5MgBr−−−−−−−−−−→2.H2OBH2SO4.Heat−−−−−−−−−→−H2OC1.O3−−−−−−−→2.Zn.H2O
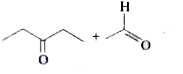
The Ketone (A) is:
View SolutionGypsum CaSO4.2H2O on heating to about 120∘ forms a compound which has the chemical composition represented by
View Solution

