Arrange the following in decreasing order of solubility in Water.
(i) Be(OH)2,Ca(OH)2,Ba(OH)2,Sr(OH)2.
(ii) BaSO4,MgSO4,CaSO4,SrSO4.
Step by step video & image solution for Arrange the following in decreasing order of solubility in Water. (i) Be(OH)_(2),Ca(OH)_(2),Ba(OH)_(2),Sr(OH)_(2). (ii) BaSO_(4), MgSO_(4), CaSO_(4), SrSO_(4). by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Book:DINESH PUBLICATIONChapter:S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Exercise:SAQ
Explore 30 Videos
- S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Book:DINESH PUBLICATIONChapter:S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Exercise:LAQ
Explore 6 Videos
Similar Questions
Knowledge Check
The decreasing order of solubility of alkaline earth metal hydroxides (M(OH)2) is
ABe<Mg<Ca<Sr<BaBBa>Sr>Ca>Mg>BeCMg<Be<Ca<BA<SrDBe=Mg>Ca>Sr>BaArrange the following groups in decreasing activating order :
(i) −NR2
(ii) −Me
(iii) NHAc
(iv) −OHAI>IV>III>IIBII>IV>III>ICIII>II>IV>IDIV>I>III>II
How do the basicity and solubility in water vary from Be(OH)2 to Ba(OH)2 ?
View SolutionArrange the following in decreasing order of their acidic character:
(i) p−CH3O−C6H4OH,
(ii) p−NO2−C6H4OH,
(iii) C6H5OH
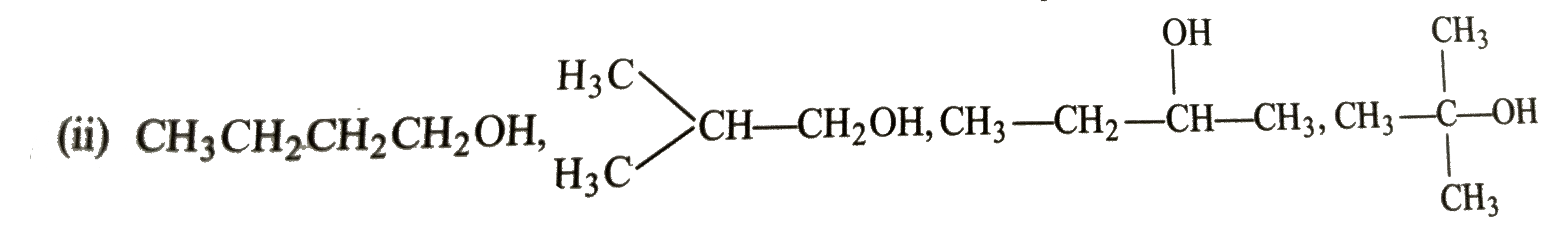
View SolutionArrange the following compounds in decreasing order fo solubility and boiling point :
(i) CH3CH2OH,CH3CH2CH2OH,CH3CH2CH2CH2OH
View SolutionHow many of the following hydroxides are soluble in excess NaOH solution?
Fe(OH)3,Al(OH)3,Zn(OH)3,Zn(OH)2,Ni(OH)2,Mn(OH)2,Sn(OH)2,Sn(OH)2,Cu(OH)2
View SolutionArrange the following as indicated:
(i) BeSO4, MgSO4.. CaSO4. SrSO4, BaSO4(increasing order of solubility)
(ii) Be(OH)2, Mg(OH)2 Ca(OH)2(increasing of solubility)
(III) BeCO3. MgCO3 CaCO3, (increasing stability)
(iv) BeCl2, MgCl2, CaCl2, SrCl2 (increasing lattice energy)
(v) BeCl2, MgCl2, CaCl2, SrCI2 (increasing order of hydrolysis)
View SolutionArrangeCaSO4, MgSO4, BaSO_(4) in increasing solubility in water.
View Solution
