There are two H-bridge bonds in diborane molecule because there are
A
four bridged hydrogens and two terminal hy drogens are present
B
Two bridged hydrogens and four terminal hydrogens
C
Three bridged hydrogens and three terminal hydrogens are present.
D
None of the above.
Video Solution
Text Solution
The correct Answer is:B
Dibronae has two bridged hydrogen and four terminal hydrogen.
|
Similar Questions
Knowledge Check
The H-O-H bond in water molecule is
A104∘31'B120∘C180∘D109∘28'The H—O—H bond in water molecule is:
A104∘31'B120∘C180∘D109∘28'The number of hydrogen atoms bridging the boron atoms in a diborane molecule is
A1B2C3D6
N- H Bond in Ammonia molecule is
View SolutionThe bond order of H2 molecule is
View SolutionThe number of three centre two electron bonds in a molecule of diborane is________.
View SolutionThe molecular shapes of diborane is shown below :
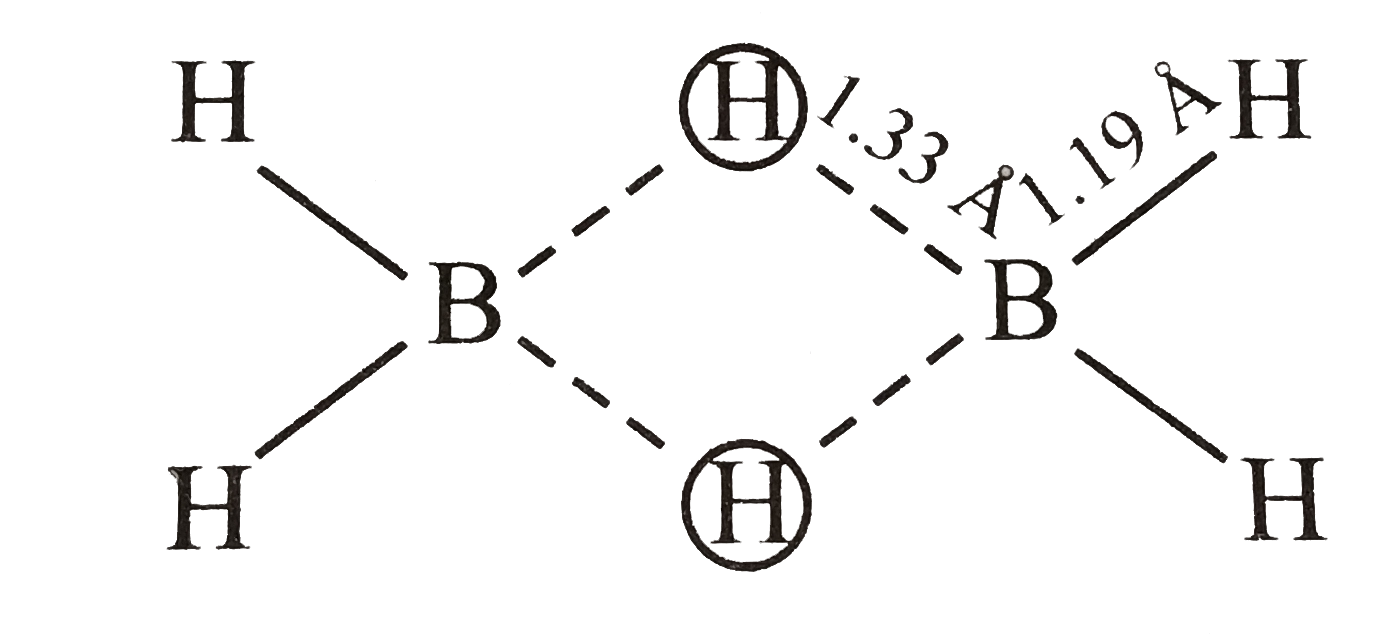 .
.
Consider the following statements for diborane :
(i) Boron is approximately sp3 hybridised.
(ii) B−H−B angle is 180∘
(iii) There are two terminal B−H bonds for each boron atom.
(iv) There are only 12 bonding electrons available of These statements :
View SolutionBond angle in H2S molecule is
View Solution

