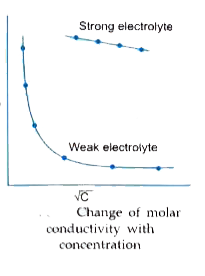
Define molar conductivity of a solution and explain how molar conductivity changes with change in concentration of a solution for a weak and a strong electrolyte.
For answer, consult section 19.
Similar Questions
(a) Define molar conductivity of a solution and explain how molar conductivity changes with change in concentration of solution for a weak and a strong electrolyte.
(b) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500Ω. What is the cell constant if the conductivity of 0.001 M KCl solution at 298 K is 0.146×10−3Scm−1?
View Solution(a) Define molar conductivity of a solution and explain how molar conductivity changes with change in concentration of solution for a weak and a strong electrolyte.
(b) The resistance of conductivity cell containing 0.001MKCl solution at 298K is 1500ω.
What is the cell constant if the conductivity of 0.001MKCl solution at 298K is 0.146×10−3Scm−1
View Solution
Knowledge Check
Molar conductivity of inic solution depends on ........
AtemperatureBdistance between electrodesCconcentration of electrolysis in solutionDsurface area of electrodesThe molar conductivity of strong electrolyte
AIncreases on dilution slightlyBDoes not change on dilutionCDecreases on dilutionDDepends on density of electrolyte itselfConductance of a solution of a strong electrolyte is measure of its
Apotential differenceBconcentrationCreactanceDdissociation
(a) Define molar conductivity of a substance and describe how for weak and strong electrolytes , molar conductivity changes with concentration of solute . How is such change explained ?
(b) A voltaic cell is set up at 25∘C with the following half cells :
Ag+(0.001M) | Ag and Cu2+(0.10M)∣Cu
What would be the voltage of this cell ? (E∘cell=0.46V)
View SolutionDefine molar conductivity of a substance and describe how for weak and strong electrolytes, molar conductivity changes with concentration of solute. How is such change explained ? A voltaic cell is set up at 25 °C with the following half-cells :
Ag+(0.001M)∣Ag and Cu2+(0.10M)∣Cu
What would be the voltage of this cell ? [E∘cell = 0.46 V]
View SolutionConductivity decreases but molar conductivity increases with ________ in concentration.
View SolutionDefine limiting molar conductivity. Why does conductivity of an electrolyte decrease with discrease in concentration.
View SolutionExplain the variation of molar conductivity with concentration for strong and weak electrolytes.
View Solution

